Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway - PubMed (original) (raw)
Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway
Nam-Gyun Kim et al. J Cell Biol. 2015.
Abstract
The Hippo pathway is involved in the regulation of contact inhibition of proliferation and responses to various physical and chemical stimuli. Recently, several upstream negative regulators of Hippo signaling, including epidermal growth factor receptor ligands and lysophosphatidic acid, have been identified. We show that fibronectin adhesion stimulation of focal adhesion kinase (FAK)-Src signaling is another upstream negative regulator of the Hippo pathway. Inhibition of FAK or Src in MCF-10A cells plated at low cell density prevented the activation of Yes-associated protein (YAP) in a large tumor suppressor homologue (Lats)-dependent manner. Attachment of serum-starved MCF-10A cells to fibronectin, but not poly-d-lysine or laminin, induced YAP nuclear accumulation via the FAK-Src-phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) signaling pathway. Attenuation of FAK, Src, PI3K, or PDK1 activity blocked YAP nuclear accumulation stimulated by adhesion to fibronectin. This negative regulation of the Hippo pathway by fibronectin adhesion signaling can, at least in part, explain the effects of cell spreading on YAP nuclear localization and represents a Lats-dependent component of the response to cell adhesion.
© 2015 Kim and Gumbiner.
Figures
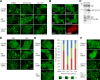
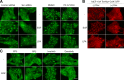
Figure 1.
PI3K, PDK1, and Src regulation of nuclear YAP via Lats in serum-starved, subconfluent cells. (A) PI3K and PDK1 inhibitors relative to Lats. MCF-10A cells transfected with control, Nf2, or Lats1/2 siRNAs were serum starved and treated with DMSO (solvent control), 10 µM wortmannin (PI3K inhibitor), or 5 µM BX-795 (PDK1 inhibitor) for 30 min. On-target plus nontargeting pool was used as a control siRNA. Localization of endogenous YAP was identified by immunofluorescence staining. (B) SFK inhibitors and YAP localization. Serum-starved, low cell density MCF-10A cells were incubated with SFK inhibitors (10 µM each of PP2, dasatinib, SKI-1, and SU6656) for 30 min. 10 µM each of PP3 and imatinib were used as controls. YAP subcellular localization was determined by immunofluorescence staining. Alexa Fluor 594 secondary antibody was used for SU6656, which has high background green fluorescence. (C) Biochemical effects of Src inhibition. PP3- or PP2-treated MCF-10A cells were analyzed by Western blot using anti-YAP and anti–phospho-YAP (S127) antibodies. Phosphorylated YAP was detected by mobility shift on Phos-tag SDS-PAGE. (D) SFK inhibitors relative to Lats. MCF-10A cells transfected with control, Nf2, or Lats1/2 siRNA were serum starved and treated with 10 µM PP3 or PP2. After 30 min, cells were fixed for immunofluorescence staining with anti-YAP antibody. (E) Depletion of individual SFK. MCF-10A cells were transfected with control, Src, Fyn, or Yes siRNA. After serum starvation, subcellular localization of endogenous YAP was identified by immunofluorescence staining and quantified based on the criteria shown under the graph. More than 120 cells from four random views were quantified. (F) Src knockdown relative to Lats. MCF-10A cells were transfected with control, Src, Lats1/2, or combined siRNA of Src and Lats1/2. Cells were serum starved for 24 h before fixation and stained with anti-YAP antibody. (A, B, and D–F) One of three independent results is presented. Bars, 25 µm.
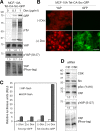
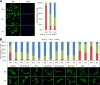
Figure 2.
Activation of Src increases YAP nuclear activity. (A) Biochemical effects of Src expression. Doxycycline-inducible expression of constitutively activated chicken-Src (Y527F, CA-Src)-GFP fusion protein in MCF-10A cells. Cells were treated with the indicated amount of doxycycline (Dox) for 12 h in complete medium and serum starved for an additional 24 h in the presence of doxycycline. Cells were lysed and subjected to Western blot analysis with the indicated antibodies. We performed two independent experiments. Tet, tetracycline (or doxycycline) inducible. (B) CA-Src expression and YAP localization. Doxycycline-inducible CA-Src-GFP–expressing MCF-10A cells were treated with 1 µg/ml doxycycline for 12 h and serum starved for 24 h in starvation medium containing doxycycline. Cells were fixed and immunofluorescence stained with anti-YAP antibody. Induction and membrane localization of CA-Src-GFP fusion protein were detected as green fluorescence. The results represent at least three independent experiments. (C) Reporter assay. Doxycycline-inducible HEK-293T cells expressing myr-GFP or CA-Src-GFP were transfected with HIP-flash or HOP-flash reporters. Luciferase activity was measured in the absence or presence of doxycycline. Data were obtained from three independent experiments. Error bars represent standard deviation. (D) Biochemical effects of CSK depletion. MCF-10A cells transfected with control (Ctrl) or CSK siRNAs were serum starved for 24 h. Cell lysates were resolved in regular or Phos-tag SDS-PAGE gels and subjected to Western blotting with the indicated antibodies. Bar, 25 µm.
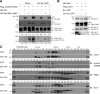
Figure 3.
Regulation of Lats1 and the Hippo complex by Src activity. (A) Inhibition of Mst2-dependent phosphorylation of Lats1 by active Src. HEK-293T cells expressing the indicated constructs were harvested at 24 h after transfection. Exogenous Flag-Lats1 protein was immunoprecipitated using anti-flag affinity gels and subjected to Western blotting with the indicated antibodies. Blots represent three independent results. (B) Src disrupts Sav1 binding to Lats1. Exogenous protein-expressing HEK-293T cells were harvested in NP-40 buffer. Sav1–Lats1 complex was coimmunoprecipitated using an anti-HA antibody. One of three independent results is presented. (C) Size-exclusion chromatography. Doxycycline-inducible TurboRFP- or myr-CSK-GFP–expressing MCF-10A cells in low cell density (∼30%) were treated with 2 µg/ml doxycycline for 12 h in complete medium and starved for 24 h before harvest. Cytosolic proteins were fractionated by HPLC gel filtration chromatography on a Superose 12 column. Each fraction and corresponding input samples (Inp) were analyzed by SDS-PAGE and Western blotting with the indicated antibodies. IB, immunoblot; IP, immunoprecipitation; WCL, whole cell lysate.
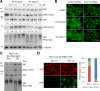
Figure 4.
FAK regulates the phosphorylation and subcellular localization of YAP via Lats kinases. (A) Dose dependence of FAK inhibitors. Serum-starved, subconfluent MCF-10A cells were incubated for 30 min at the indicated concentrations of the two FAK inhibitors. Cell lysates were separated by SDS-PAGE or Phos-tag SDS-PAGE gels and immunoblotted with the indicated antibodies. γ-Tubulin was used as a loading control. We performed two independent experiments. (B) FAK inhibitors and YAP localization. MCF-10A cells transfected with control or Lats1/2 siRNA were serum starved and treated with DMSO, 5 µM PF-573228, or 10 µM PF-562271 for 30 min. Endogenous YAP was immunofluorescence stained with anti-YAP antibody. One of three independent results is presented. (C) Biochemical effects of FRNK expression. Doxycycline-inducible FRNK-GFP–expressing MCF-10A cells were treated with 1 µg/ml doxycycline (Dox) for 12 h and serum starved for 24 h in starvation medium containing doxycycline. Cells were detached, held in suspension for 30 min (Sus), and then replated on fibronectin (FN)-coated coverslips in starvation medium for 2 h. The result shown is one of two independent results. (D) FRNK expression and YAP localization. MCF-10A cells were either uninduced or induced to express FRNK-GFP with 1 µg/ml doxycycline for 12 h and serum starved for 24 h in starvation medium containing doxycycline. Cells were dissociated and seeded on fibronectin-coated coverslips in starvation medium. Induction of FRNK-GFP and localization to focal adhesions were detected as green fluorescence. More than 150 cells from four random views were quantified, and data represent one of three independent results. Tet, tetracycline (or doxycycline) inducible. Bars, 25 µm.
Figure 5.
PI3K–PDK1, but not FAK, act downstream of Src. MCF-10A cells expressing doxycycline-inducible CA-Src-GFP fusion protein were treated with 1 µg/ml doxycycline (Dox) in complete medium for 12 h and starved for 24 h in starvation medium containing doxycycline. Indicated inhibitors were added to culture medium and incubated for 30 min before fixation. Subcellular localization of YAP was determined by immunofluorescence staining. One of three independent results is presented. Tet, tetracycline (or doxycycline) inducible. Bar, 25 µm.
Figure 6.
Roles of Src and FAK in the regulation of nuclear YAP by EGF or LPA. (A) Src depletion and FAK inhibition. MCF-10A cells transfected with control or Src siRNA were serum starved for 24 h and treated with EGF or LPA for 30 min. For FAK inhibitor treatment experiment, serum-starved, subconfluent MCF-10A cells were pretreated for 30 min with DMSO or 5 µM PF-573228, followed by a 30-min EGF or LPA treatment. YAP subcellular localization was determined by immunofluorescence staining. One of three independent results is presented. (B) CSK expression to inhibit Src activity. Paired with uninduced cells, doxycycline (Dox)-induced MCF-10A cells expressing myr-CSK-GFP fusion protein were serum starved for 24 h. Cells were treated with EGF or LPA for 30 min before fixation. Subcellular localization of YAP was determined by immunofluorescence staining. One representative out of three independent experiments is shown. Tet, tetracycline (or doxycycline) inducible. (C) Src inhibitors. Serum-starved, subconfluent MCF-10A cells were pretreated with PP3, PP2, imatinib, or dasatinib for 30 min. Cells were then treated with 20 ng/ml EGF for 30 min before fixation. Endogenous YAP was immunofluorescence stained. The result represents three independent experiments. Bars, 25 µm.
Figure 7.
Attachment to fibronectin-coated coverslips induces YAP nuclear accumulation via the FAK–Src–PI3K–PDK1 pathway. (A) Attachment to fibronectin, poly-
d
-lysine, or laminin. Serum-starved MCF-10A cells were dissociated with Accutase and sparsely seeded on fibronectin (FN)-, poly-
d
-lysine (PL)–, or laminin (LN)-coated coverslips in starvation medium. Cells were incubated for 2 h before fixation. Subcellular localization of YAP was identified by immunofluorescence staining and quantified based on the criteria shown in Fig. 1 E. More than 170 cells from eight random views were quantified. DAPI staining was used to locate the nucleus. We performed three independent experiments. (B) Effects of inhibitors and growth factors on attachment-induced YAP nuclear localization. Serum-starved MCF-10A cells were detached and seeded on fibronectin- or poly-
d
-lysine–coated coverslips in starvation medium. After 3 h of incubation, cells were treated with the indicated inhibitors or mitogens for 30 min. Subcellular localization of YAP was identified by immunofluorescence staining. More than 120 cells from eight random views were quantified and confirmed in three independent experiments. Bars, 25 µm.
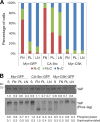
Figure 8.
Role of Src in the regulation of YAP by ECM adhesion. (A) Effects of Src on cell–matrix adhesion regulation of YAP subcellular localization. Doxycycline-inducible myr-GFP– (control), CA-Src-GFP–, or myr-CSK-GFP–expressing MCF-10A cells were treated with 1 µg/ml doxycycline for 12 h and maintained in starvation medium containing doxycycline for an additional 24 h. Cells were dissociated and seeded on fibronectin (FN)-, poly-
d
-lysine (PL)–, or laminin (LN)-coated coverslips in starvation medium. Subcellular localization of YAP was identified by immunofluorescence staining, and >200 cells from eight random views were quantified and confirmed in two independent experiments. (B) Effects of myr-GFP, CA-Src-GFP, and myr-CSK-GFP expression on YAP phosphorylation. Cells described in A were lysed with 2× SDS buffer and separated by SDS-PAGE or Phos-tag SDS-PAGE gels and immunoblotted with anti-YAP antibody.
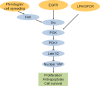
Figure 9.
Upstream negative regulators of the Hippo pathway. Soluble growth factors including EGF and LPA inactivate the growth-inhibitory Hippo pathway through the stimulation of PI3K–PDK1 signaling. EGFR, but not LPA, signaling depends on Src kinase. Integrin receptors bind to fibronectin (FN), and mechanical stimulus triggers FAK-Src signaling and leads to the activation of YAP in a PI3K–PDK1-dependent manner.
Similar articles
- Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1.
Fan R, Kim NG, Gumbiner BM. Fan R, et al. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2569-74. doi: 10.1073/pnas.1216462110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359693 Free PMC article. - Arterial Wall Stress Induces Phenotypic Switching of Arterial Smooth Muscle Cells in Vascular Remodeling by Activating the YAP/TAZ Signaling Pathway.
Wang Y, Cao W, Cui J, Yu Y, Zhao Y, Shi J, Wu J, Xia Z, Yu B, Liu J. Wang Y, et al. Cell Physiol Biochem. 2018;51(2):842-853. doi: 10.1159/000495376. Epub 2018 Nov 22. Cell Physiol Biochem. 2018. PMID: 30466081 - Caveolin-1 and integrin β1 regulate embryonic stem cell proliferation via p38 MAPK and FAK in high glucose.
Lee SH, Lee YJ, Park SW, Kim HS, Han HJ. Lee SH, et al. J Cell Physiol. 2011 Jul;226(7):1850-9. doi: 10.1002/jcp.22510. J Cell Physiol. 2011. PMID: 21506116 - Src kinase: Key effector in mechanosignalling.
Koudelková L, Brábek J, Rosel D. Koudelková L, et al. Int J Biochem Cell Biol. 2021 Feb;131:105908. doi: 10.1016/j.biocel.2020.105908. Epub 2020 Dec 25. Int J Biochem Cell Biol. 2021. PMID: 33359015 Review. - Ste20-like kinase SLK, at the crossroads: a matter of life and death.
Al-Zahrani KN, Baron KD, Sabourin LA. Al-Zahrani KN, et al. Cell Adh Migr. 2013 Jan-Feb;7(1):1-10. doi: 10.4161/cam.22495. Epub 2012 Nov 15. Cell Adh Migr. 2013. PMID: 23154402 Free PMC article. Review.
Cited by
- P4HA2 knockdown prevents the progression of intracranial aneurysm by inducing prolyl hydroxylation of YAP1.
Li L, Wang J, Ren S, Hao X. Li L, et al. Neurosurg Rev. 2024 Nov 19;47(1):858. doi: 10.1007/s10143-024-03101-9. Neurosurg Rev. 2024. PMID: 39560705 - Avian Pasteurella multocida induces chicken macrophage apoptosis by inhibiting the Zyxin-FAK-AKT-FoxO1/NF-κB axis.
Li P, Zhao G, Tang T, He F, Liu X, Li N, Peng Y. Li P, et al. Poult Sci. 2024 Dec;103(12):104504. doi: 10.1016/j.psj.2024.104504. Epub 2024 Nov 2. Poult Sci. 2024. PMID: 39510005 Free PMC article. - IFN-γ and YAP lead epithelial cells astray after severe respiratory infection.
Hiller BE, Mizgerd JP. Hiller BE, et al. J Clin Invest. 2024 Oct 1;134(19):e185072. doi: 10.1172/JCI185072. J Clin Invest. 2024. PMID: 39352386 Free PMC article. - Viral infection induces inflammatory signals that coordinate YAP regulation of dysplastic cells in lung alveoli.
Lin X, Chen W, Yang G, Zhang J, Wang H, Liu Z, Xi Y, Ren T, Liu B, Sui P. Lin X, et al. J Clin Invest. 2024 Oct 1;134(19):e176828. doi: 10.1172/JCI176828. J Clin Invest. 2024. PMID: 39352385 Free PMC article. - Identifying adeno-associated virus (AAV) vectors that efficiently target high grade glioma cells, for in vitro monitoring of temporal cell responses.
Sarker FA, Chen Y, Westhaus A, Lisowski L, O'Neill GM. Sarker FA, et al. FEBS Open Bio. 2024 Nov;14(11):1914-1925. doi: 10.1002/2211-5463.13894. Epub 2024 Sep 10. FEBS Open Bio. 2024. PMID: 39256894 Free PMC article.
References
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., and Sahai E.. 2013. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15:637–646. 10.1038/ncb2756 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous