Therapeutic Effect of Berberine on Huntington's Disease Transgenic Mouse Model - PubMed (original) (raw)
Therapeutic Effect of Berberine on Huntington's Disease Transgenic Mouse Model
Wenxiao Jiang et al. PLoS One. 2015.
Abstract
Huntington disease (HD) represents a family of neurodegenerative diseases that are caused by misfolded proteins. The misfolded proteins accumulate in the affected brain regions in an age-dependent manner to cause late-onset neurodegeneration. Transgenic mouse models expressing the HD protein, huntingtin, have been widely used to identify therapeutics that may retard disease progression. Here we report that Berberine (BBR), an organic small molecule isolated from plants, has protective effects on transgenic HD (N171-82Q) mice. We found that BBR can reduce the accumulation of mutant huntingtin in cultured cells. More importantly, when given orally, BBR could effectively alleviate motor dysfunction and prolong the survival of transgenic N171-82Q HD mice. We found that BBR could promote the degradation of mutant huntingtin by enhancing autophagic function. Since BBR is an orally-taken drug that has been safely used to treat a number of diseases, our findings suggest that BBR can be tested on different HD animal models and HD patients to further evaluate its therapeutic effects.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
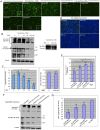
Fig 1. BBR reduced Htt aggregation in vitro in a dose and time-dependent manner.
(A) Immunocytostaining images (10 X) of Htt-120Q- or Htt-20Q-transfected HEK293 cells that were treated with different concentrations of BBR (0, 5, 25, 50, 100 μM). (B) Western blot analysis of Htt-transfected cells treated with or without BBR at different concentrations. Aggregated Htt in the stacking gel was detected by mEM48 antibody. Soluble mutant Htt and its potential degraded products are also shown. (C) Densitometry analysis of the ratios of aggregated Htt or Htt-20Q to β-actin on western blots in (B). (D) Fluorescent microscopic images of Htt-120Q-transfected HEK293 cells that were treated with 50 μM BBR at 0 h, 12 h or 24 h post-transfection. (E) Cell-counting analysis of images obtained in (D) showing the percentage of aggregates relative to the total cells revealed by DAPI nuclear staining. (F) Western blotting of Htt-120Q transfected HEK293 cells treated with 50 μm BBR for different times showing aggregated Htt in the stacking gel. (G) Densitometry analysis of the ratios of mutant Htt to β-actin on western blots in (F). The quantitative data are presented as mean±SE.
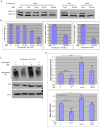
Fig 2. BBR increases autophagic activity in cultured cells.
(A) Western blotting of LC3B in HEK293 cells transfected with or without Htt and treated with BBR (0, 5, 25, 50, or 100 μM). (B) Densitometry analysis of the ratios of LC3-I to LC3-II in above Western blots. (C) Western blotting of Htt-transfected HEK293 cells treated with or without 50 μM BBR or bafilomycin (BFA), an autophagy inhibitor. Antibody to P62, which decreases when autophagy activates, was used to confirm the altered activity of autophagy. (D) Densitometry analysis of the ratios of aggregated Htt or P62 to actin in Western blots in (C). The quantitative data are presented as mean±SE.
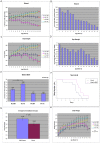
Fig 3. Oral administration of BBR ameliorates neurological symptoms in transgenic N171-82Q mice.
(A, B) Rotarod test of motor coordination of N171-82Q transgenic mice and WT siblings, orally gavaged with 40 mg/kg BBR or vehicle daily. (C, D) Grip strength test on transgenic N171-82Q mice and WT siblings treated with BBR or vehicle. (E) Balance beam test on 18-week-old N171-82Q and WT mice that had been treated with BBR or vehicle. (F) Survival curve for N171-82Q mice and WT siblings treated with BBR or vehicle. (G) The BBR-treated N171-82Q group survived 15 days longer than their untreated HD siblings. (H) Body weight of N171-82Q mice and WT siblings treated with or without BBR. The quantitative data are presented as mean±SE. *p<0.05. **p<0.01. ***p<0.001, n = 7 mice per group.
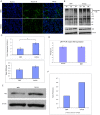
Fig 4. Oral administration of BBR reduced mutant Htt aggregation and increased autophagy in transgenic N171-82Q mice.
(A) Fluorescent immunohistostaining of mouse brain slices from transgenic N171-82Q HD mice and WT siblings, orally gavaged with 40 mg/kg BBR or vehicle daily. (B) Western blotting of the cortex samples of 3 mice per group (HD+BBR vs. HD+vehicle) showing mutant Htt aggregates in the stacking gel and P62. Non-specific immunoreactive bands are also shown on the blots. Actin was used to show the internal control protein on western blot. (C) Densitometry analysis of above Western blots. The data are mean±SE (n = 3). (D) Quantitative RT-PCR analysis of transgenic mutant Htt mRNA in the cortex of the BBR-treated or untreated N171-82Q mice. n = 3 mice per group. Data are presented as mean CT±SEM. (E) Western blotting of WT mouse cortex samples (3-mouse pool per group) after the mice had been orally gavaged with 40 mg/kg BBR or vehicle daily for 24 weeks and sacrificed 4 h after the last BBR administration. (F) Densitometry analysis of Western blots in (E) showing the ratio of P62 to actin. The quantitative data are presented as mean±SE.
Similar articles
- Altered selenium status in Huntington's disease: neuroprotection by selenite in the N171-82Q mouse model.
Lu Z, Marks E, Chen J, Moline J, Barrows L, Raisbeck M, Volitakis I, Cherny RA, Chopra V, Bush AI, Hersch S, Fox JH. Lu Z, et al. Neurobiol Dis. 2014 Nov;71:34-42. doi: 10.1016/j.nbd.2014.06.022. Epub 2014 Jul 8. Neurobiol Dis. 2014. PMID: 25014023 - Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington's disease.
Jiang M, Peng Q, Liu X, Jin J, Hou Z, Zhang J, Mori S, Ross CA, Ye K, Duan W. Jiang M, et al. Hum Mol Genet. 2013 Jun 15;22(12):2462-70. doi: 10.1093/hmg/ddt098. Epub 2013 Feb 27. Hum Mol Genet. 2013. PMID: 23446639 Free PMC article. - Luteolin as potential treatment for Huntington's disease: Insights from a transgenic mouse model.
Mohammed A, Ramadan A, Elnour AA, Saeed AAAM, Al Mazrouei N, Alsulami FT, Alqarni YS, Menon V, Amoodi AA, Abdalla SF. Mohammed A, et al. CNS Neurosci Ther. 2024 Sep;30(9):e70025. doi: 10.1111/cns.70025. CNS Neurosci Ther. 2024. PMID: 39228080 Free PMC article. - Transgenic animal models for study of the pathogenesis of Huntington's disease and therapy.
Chang R, Liu X, Li S, Li XJ. Chang R, et al. Drug Des Devel Ther. 2015 Apr 15;9:2179-88. doi: 10.2147/DDDT.S58470. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25931812 Free PMC article. Review. - Role of Phosphodiesterases in Huntington's Disease.
Fusco FR, Paldino E. Fusco FR, et al. Adv Neurobiol. 2017;17:285-304. doi: 10.1007/978-3-319-58811-7_11. Adv Neurobiol. 2017. PMID: 28956337 Review.
Cited by
- The Emerging Landscape of Natural Small-molecule Therapeutics for Huntington's Disease.
Bhat SA, Ahamad S, Dar NJ, Siddique YH, Nazir A. Bhat SA, et al. Curr Neuropharmacol. 2023;21(4):867-889. doi: 10.2174/1570159X21666230216104621. Curr Neuropharmacol. 2023. PMID: 36797612 Free PMC article. Review. - Demethyleneberberine, a potential therapeutic agent in neurodegenerative disorders: a proposed mechanistic insight.
Saklani P, Khan H, Singh TG, Gupta S, Grewal AK. Saklani P, et al. Mol Biol Rep. 2022 Oct;49(10):10101-10113. doi: 10.1007/s11033-022-07594-9. Epub 2022 Jun 3. Mol Biol Rep. 2022. PMID: 35657450 Review. - Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders.
Hussain G, Rasul A, Anwar H, Aziz N, Razzaq A, Wei W, Ali M, Li J, Li X. Hussain G, et al. Int J Biol Sci. 2018 Mar 9;14(3):341-357. doi: 10.7150/ijbs.23247. eCollection 2018. Int J Biol Sci. 2018. PMID: 29559851 Free PMC article. Review. - Combating Neurodegenerative Diseases with the Plant Alkaloid Berberine: Molecular Mechanisms and Therapeutic Potential.
Fan D, Liu L, Wu Z, Cao M. Fan D, et al. Curr Neuropharmacol. 2019;17(6):563-579. doi: 10.2174/1570159X16666180419141613. Curr Neuropharmacol. 2019. PMID: 29676231 Free PMC article. Review. - Molecular Insights into the Multifunctional Role of Natural Compounds: Autophagy Modulation and Cancer Prevention.
Rahman MA, Rahman MDH, Hossain MS, Biswas P, Islam R, Uddin MJ, Rahman MH, Rhim H. Rahman MA, et al. Biomedicines. 2020 Nov 19;8(11):517. doi: 10.3390/biomedicines8110517. Biomedicines. 2020. PMID: 33228222 Free PMC article. Review.
References
- Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J et al. A worldwide study of the Huntington's disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994; 330: 1401–1406. - PubMed
- Squitieri F, Frati L, Ciarmiello A, Lastoria S, Quarrell O. Juvenile Huntington's disease: does a dosage-effect pathogenic mechanism differ from the classical adult disease? Mech Ageing Dev. 2006;127: 208–212. - PubMed
- Harjes P, Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28: 425–433. - PubMed
- Li SH, Li XJ. Huntingtin-protein interactions and the pathogenesis of Huntington's disease. Trends Genet. 2004;20: 146–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AG019206/AG/NIA NIH HHS/United States
- R01 NS041669/NS/NINDS NIH HHS/United States
- AG019206/AG/NIA NIH HHS/United States
- R01 NS045016/NS/NINDS NIH HHS/United States
- R01 AG031153/AG/NIA NIH HHS/United States
- R01 NS036232/NS/NINDS NIH HHS/United States
- NS036232/NS/NINDS NIH HHS/United States
- NS041669/NS/NINDS NIH HHS/United States
- R01 AG019206/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical