Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant - PubMed (original) (raw)
Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant
Vladimir Beljanski et al. J Virol. 2015 Oct.
Abstract
The molecular interaction between viral RNA and the cytosolic sensor RIG-I represents the initial trigger in the development of an effective immune response against infection with RNA viruses, resulting in innate immune activation and subsequent induction of adaptive responses. In the present study, the adjuvant properties of a sequence-optimized 5'-triphosphate-containing RNA (5'pppRNA) RIG-I agonist (termed M8) were examined in combination with influenza virus-like particles (VLP) (M8-VLP) expressing H5N1 influenza virus hemagglutinin (HA) and neuraminidase (NA) as immunogens. In combination with VLP, M8 increased the antibody response to VLP immunization, provided VLP antigen sparing, and protected mice from a lethal challenge with H5N1 influenza virus. M8-VLP immunization also led to long-term protective responses against influenza virus infection in mice. M8 adjuvantation of VLP increased endpoint and antibody titers and inhibited influenza virus replication in lungs compared with approved or experimental adjuvants alum, AddaVax, and poly(I·C). Uniquely, immunization with M8-VLP stimulated a TH1-biased CD4 T cell response, as determined by increased TH1 cytokine levels in CD4 T cells and increased IgG2 levels in sera. Collectively, these data demonstrate that a sequence-optimized, RIG-I-specific agonist is a potent adjuvant that can be utilized to increase the efficacy of influenza VLP vaccination and dramatically improve humoral and cellular mediated protective responses against influenza virus challenge.
Importance: The development of novel adjuvants to increase vaccine immunogenicity is an important goal that seeks to improve vaccine efficacy and ultimately prevent infections that endanger human health. This proof-of-principle study investigated the adjuvant properties of a sequence-optimized 5'pppRNA agonist (M8) with enhanced capacity to stimulate antiviral and inflammatory gene networks using influenza virus-like particles (VLP) expressing HA and NA as immunogens. Vaccination with VLP in combination with M8 increased anti-influenza virus antibody titers and protected animals from lethal influenza virus challenge, highlighting the potential clinical use of M8 as an adjuvant in vaccine development. Altogether, the results describe a novel immunostimulatory agonist targeted to the cytosolic RIG-I sensor as an attractive vaccine adjuvant candidate that can be used to increase vaccine efficacy, a pressing issue in children and the elderly population.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
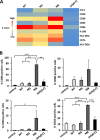
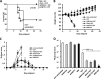
Transfecting monocyte-derived dendritic cells (MDDC) with WT, M5, or M8 5′pppRNA or poly(I·C) increases expression of activation and differentiation markers and their mRNA levels. MDDC were isolated from peripheral blood mononuclear cells (n = 4), differentiated, and transfected with 10 ng WT, M5, M8, or poly(I·C) using HiPerFect transfection reagent for 24 h. (A) Gene expression analysis using the Fluidigm BioMark platform for several genes (indicated on the right) in MDDC transfected with 20 fmol of WT, M5, M8, or poly(I·C) for 24 h. (B) MDDC surface expression of activation and differentiation markers as assessed by flow cytometry (mean plus standard error of the mean [SEM] [error bar]). Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005.
FIG 2
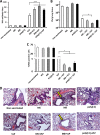
Protective efficacy of the VLP vaccine adjuvanted with M5, M8, or poly(I·C). Mice (n = 5) were immunized intramuscularly with 2 μg of VLP alone or combined with 5 μg M5, M8, or poly(I·C) as a 50-μl injection, and 3 weeks later, they were challenged with 5,000 PFU of H5N1 influenza virus. (A) Hemagglutination inhibition (HAI) antibody titers in immunized mice prior to infection were determined by hemagglutination inhibition assay using horse red blood cells. (B) Assessment of viral replication in lungs of infected animals 3 days postinfection by a plaque assay. (C) TUNEL-positive (apoptotic) lung cells in infected mice were quantified by a TUNEL assay as described in Materials and Methods. All values in panels A to C are expressed as means plus SEMs. Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; ***, P ≤ 0.005. (D) H&E staining of paraffin-embedded lung cross sections from mice 3 days after challenge. The yellow arrows indicate the airways of mice that were vaccinated with M8 only (top) or M8-VLP (bottom).
FIG 3
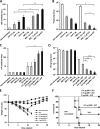
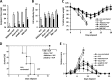
Dose-response assessment of VLP and M8. (A and B) Mice (n = 5) were immunized with decreasing doses of VLP (2 μg to 0.5 μg) in combination with 5 μg of M8; 3 weeks later, they were challenged with H5N1 influenza virus, and lungs from infected animals were harvested 3 days postchallenge. HAI antibody titers in immunized mice prior to infection were determined by HAI assay (A), and viral replication in lungs was assessed by plaque assay (B). (C to F) Mice were immunized with 0.5 μg of VLP with 0.1 to 5 μg of M8. HAI antibody titers were determined by HAI assay (C), and viral replication was determined by plaque assay (D). Weight loss (E) and survival (F) were determined as well. Values are expressed as means plus SEMs. Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005.
FIG 4
Adjuvant comparison strategy and antibody immune responses for M8, alum, AddaVax, and poly(I·C)-adjuvanted VLP vaccine. (A) Strategy for adjuvant comparison. 3d, 3 days. (B to D) Mice were immunized with 0.5 μg of VLP in combination with 5 μg of M8 or poly(I·C) or in combination with 50% volume of alum or AddaVax, and 5 days and 3 weeks after immunization, sera were collected. HA-specific IgG antibodies (B) and influenza virus HAI antibody titers (C) were determined 3 weeks after immunization by ELISA and HAI assay, respectively. (D) HA-specific IgM antibodies were determined 5 days after immunization by ELISA. Values are expressed as means plus SEMs. Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01. O.D., optical density.
FIG 5
Protective efficacy of 0.5 μg of VLP in combination with 5 μg of M8 or poly(I·C) or in combination with 50% volume of alum or AddaVax. (A to C) Three weeks after vaccination, mice (n = 8) were challenged with the lethal dose of H5N1 (5,000 PFU), and their survival (A), weight (B), and sickness score (C) were monitored. (D) Viral replication in lungs was assessed by plaque assay in a separate group of immunized animals (n = 5) 3 days postinfection. Values are expressed as means ± SEMs. Values that are significantly different (P ≤ 0.005) are indicated by bars and asterisks (***).
FIG 6
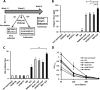
Long-term protective responses in mice immunized with 0.5 μg of VLP in combination with 5 μg of M8 or poly(I·C) or in combination with 50% volume of alum or AddaVax. Mouse sera (n = 8) were collected 4 weeks (white bars) and 16 weeks (black bars) postvaccination to determine HA-specific IgG antibodies (ELISA) (A) and HAI antibody titers (B). Upon challenge with a lethal H5N1 dose (5,000 PFU) weight (C), survival (D), and sickness score (E) were monitored for 3 weeks. Values are expressed as the means ± SEMs.
FIG 7
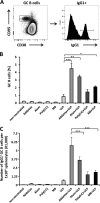
Quantification of germinal center (GC) B cells from spleens of i.m. immunized mice (n = 5) by flow cytometry. (A) Gating strategy for quantification of GC B cells; (B) percentage of GC B cells in B220hi splenocytes; (C) quantification of IgG1+ GC B cells. Values are expressed as means plus SEMs. Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005.
FIG 8
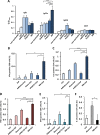
Quantification of IgG subclasses from i.m. vaccinated animals (n = 8) and intracellular cytokine levels in T cells isolated from the spleens of i.p. vaccinated animals (n = 5) after 24 h of VLP stimulation. (A) IgG subclasses, IgG1, IgG2a, IgG2b, and IgG3, from sera of vaccinated animals (as indicated below the bars) were determined by ELISA using HA-coated plates. (B to F) The percentages of cytokine-secreting cells were obtained by subtracting the numbers of cytokine-secreting cells that were not stimulated with VLP. The percentages of IFN-γ+ CD8hi cells (B), IL-2+ CD4hi cells (C), TNF-α+ CD4hi cells (D), IFN-γ+ CD4hi cells (E), and IL-10+ CD4hi cells (F) are shown. All values are expressed as means plus SEMs. Values that are significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005.
Similar articles
- Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists.
Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK, Trautmann L, Ross T, Lin R, Hiscott J. Chiang C, et al. J Virol. 2015 Aug;89(15):8011-25. doi: 10.1128/JVI.00845-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018150 Free PMC article. - Plant-derived H7 VLP vaccine elicits protective immune response against H7N9 influenza virus in mice and ferrets.
Pillet S, Racine T, Nfon C, Di Lenardo TZ, Babiuk S, Ward BJ, Kobinger GP, Landry N. Pillet S, et al. Vaccine. 2015 Nov 17;33(46):6282-9. doi: 10.1016/j.vaccine.2015.09.065. Epub 2015 Oct 2. Vaccine. 2015. PMID: 26432915 - Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge.
Galarza JM, Latham T, Cupo A. Galarza JM, et al. Viral Immunol. 2005;18(1):244-51. doi: 10.1089/vim.2005.18.244. Viral Immunol. 2005. PMID: 15802970 - Virus-like particles as universal influenza vaccines.
Kang SM, Kim MC, Compans RW. Kang SM, et al. Expert Rev Vaccines. 2012 Aug;11(8):995-1007. doi: 10.1586/erv.12.70. Expert Rev Vaccines. 2012. PMID: 23002980 Free PMC article. Review. - Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.
Nagashima KA, Mousa JJ. Nagashima KA, et al. Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review.
Cited by
- Progress in the development of virus-like particle vaccines against respiratory viruses.
Quan FS, Basak S, Chu KB, Kim SS, Kang SM. Quan FS, et al. Expert Rev Vaccines. 2020 Jan;19(1):11-24. doi: 10.1080/14760584.2020.1711053. Epub 2020 Jan 18. Expert Rev Vaccines. 2020. PMID: 31903811 Free PMC article. Review. - Structural Variability in the RLR-MAVS Pathway and Sensitive Detection of Viral RNAs.
Jiang QX. Jiang QX. Med Chem. 2019;15(5):443-458. doi: 10.2174/1573406415666181219101613. Med Chem. 2019. PMID: 30569868 Free PMC article. Review. - RIG-I-like receptor activation by dengue virus drives follicular T helper cell formation and antibody production.
Sprokholt JK, Kaptein TM, van Hamme JL, Overmars RJ, Gringhuis SI, Geijtenbeek TBH. Sprokholt JK, et al. PLoS Pathog. 2017 Nov 29;13(11):e1006738. doi: 10.1371/journal.ppat.1006738. eCollection 2017 Nov. PLoS Pathog. 2017. PMID: 29186193 Free PMC article. - The impact of immuno-aging on SARS-CoV-2 vaccine development.
Connors J, Bell MR, Marcy J, Kutzler M, Haddad EK. Connors J, et al. Geroscience. 2021 Feb;43(1):31-51. doi: 10.1007/s11357-021-00323-3. Epub 2021 Feb 11. Geroscience. 2021. PMID: 33569701 Free PMC article. Review. - HIF-1α promotes virus replication and cytokine storm in H1N1 virus-induced severe pneumonia through cellular metabolic reprogramming.
Meng X, Zhu Y, Yang W, Zhang J, Jin W, Tian R, Yang Z, Wang R. Meng X, et al. Virol Sin. 2024 Feb;39(1):81-96. doi: 10.1016/j.virs.2023.11.010. Epub 2023 Nov 30. Virol Sin. 2024. PMID: 38042371 Free PMC article.
References
- Metcalf TU, Cubas RA, Ghneim K, Cartwright MJ, Grevenynghe JV, Richner JM, Olagnier DP, Wilkinson PA, Cameron MJ, Park BS, Hiscott JB, Diamond MS, Wertheimer AM, Nikolich-Zugich J, Haddad EK. 2015. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14:421–432. doi:10.1111/acel.12320. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials