FUS Interacts with HSP60 to Promote Mitochondrial Damage - PubMed (original) (raw)
. 2015 Sep 3;11(9):e1005357.
doi: 10.1371/journal.pgen.1005357. eCollection 2015 Sep.
Mengxue Yang 2, Yanbo Chen 3, Xiaoping Chen 4, Jianghong Liu 5, Shufeng Sun 5, Haipeng Cheng 4, Yang Li 6, Eileen H Bigio 7, Marsel Mesulam 7, Qi Xu 8, Sidan Du 9, Kazuo Fushimi 4, Li Zhu 5, Jane Y Wu 10
Affiliations
- PMID: 26335776
- PMCID: PMC4559378
- DOI: 10.1371/journal.pgen.1005357
FUS Interacts with HSP60 to Promote Mitochondrial Damage
Jianwen Deng et al. PLoS Genet. 2015.
Abstract
FUS-proteinopathies, a group of heterogeneous disorders including ALS-FUS and FTLD-FUS, are characterized by the formation of inclusion bodies containing the nuclear protein FUS in the affected patients. However, the underlying molecular and cellular defects remain unclear. Here we provide evidence for mitochondrial localization of FUS and its induction of mitochondrial damage. Remarkably, FTLD-FUS brain samples show increased FUS expression and mitochondrial defects. Biochemical and genetic data demonstrate that FUS interacts with a mitochondrial chaperonin, HSP60, and that FUS translocation to mitochondria is, at least in part, mediated by HSP60. Down-regulating HSP60 reduces mitochondrially localized FUS and partially rescues mitochondrial defects and neurodegenerative phenotypes caused by FUS expression in transgenic flies. This is the first report of direct mitochondrial targeting by a nuclear protein associated with neurodegeneration, suggesting that mitochondrial impairment may represent a critical event in different forms of FUS-proteinopathies and a common pathological feature for both ALS-FUS and FTLD-FUS. Our study offers a potential explanation for the highly heterogeneous nature and complex genetic presentation of different forms of FUS-proteinopathies. Our data also suggest that mitochondrial damage may be a target in future development of diagnostic and therapeutic tools for FUS-proteinopathies, a group of devastating neurodegenerative diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
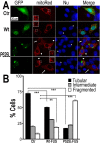
Fig 1. Expression of Wt- or ALS-mutant FUS in HT22 neuron-like cells led to mitochondrial fragmentation.
**(A)**The mitoRed plasmid was co-transfected together with plasmids expressing either the control GFP vector (Ctr), or Wt- or P525L-mutant FUS into HT22 cells. Cells with tubular, intermediate or fragmented mitochondrial patterns were imaged 72 hrs post-transfection and quantified. Arrowheads mark the cells that expressed exogenous FUS and showed fragmented mitochondria (with their nuclei marked by “*”); whereas the arrows mark adjacent non-transfected cells showing tubular mitochondria. Insets show the boxed areas at a higher magnification.(B)Quantification of mitochondrial fragmentation from experiments shown in panel A. The data were analyzed using one-way ANOVA with Bonferroni post-test (**: p<0.001; ***: p<0.0001).
Fig 2. Expression of Wt- or P525L- mutant FUS in primary cortical neurons (A-C) or fly motor neurons (D-F)led to mitochondrial fragmentation.
The mitoRed plasmid was co-transfected together with plasmids expressing either the control GFP vector (Ctr), or Wt- or P525L-mutant FUS into E18 murine cortical neurons(A-C)with quantification of apoptotic neurons containing condensed nuclei as previously published [94], shown in panel B and C respectively. In panel A, arrows label neurons showing normal mitochondria, whereas the arrowheads mark neurons showing fragmented mitochondria. The “*” labels nuclei with normal morphology, whereas “#” mark the condensed or fragmented nuclei (signs of apoptosis). The higher magnification images of the boxed areas are shown in insets at the bottom of the first set of images in panel A. (D-F). FUS expression in fly motor neurons (MNs) led to mitochondrial fragmentation. In the axonal bundles of MNs, mitochondria were visualized by mitoGFP expression in the 3rd instar larvae. (D) Confocal microscopic images of motor neuron axons in the A3 abdominal segment were obtained in Z-stacks and projected into single images, as described previously [90]. Fly genotypes, Ctr: D42-Gal4/UAS-mitoGFP/UAS-RFP; Wt: D42-Gal4/UAS-mitoGFP/UAS-Wt-FUS-RFP; P525L: D42-Gal4/UAS-mitoGFP/UAS-P525L-FUS-RFP. (E) Quantification of mitochondrial size distribution using Image J. The Wt- or P525L-mutant FUS expressing flies showed significantly increased numbers of smaller mitochondria, but fewer mitochondria with larger sizes as compared with the control group. Ten larvae were quantified in each group. (F) Quantification of mitochondrial lengths in MNs axons. The 3rd instar larvae expressing Wt- or P525L-mutant FUS showed significantly smaller mitochondria as compared with the control flies. Ten larvae were measured in each group. All data were analyzed using one-way ANOVA with Bonferronipost-test (*: p<0.05; **: p<0.01; ***: p<0.0001).
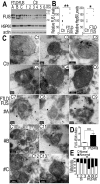
Fig 3. The FUS protein is associated with mitochondria.
(A)Highly purified mitochondria were prepared from the control or P525L-FUS-expressing stable HEK cell lines. The mitochondrial purity was confirmed by the detection of mitochondrial CoxIV and the absence of the cytoplasmic proteins such as GAPDH or nuclear protein Histone H3. The endogenous FUS or P525L-mutant FUS localized to mitochondria; and the P525L-mutant FUS showed increased levels of mitochondrial localization (lane 6), as compared with the endogenous FUS in mitochondria (lane 3). (B-D) IEM images of the control or FUS-expressing stable HEK cell lines show reduced mitochondrial sizes in cells overexpressing FUS. Arrows, FUS-immunostaining signals associated with mitochondria labeled with 10-nm immuno-gold particles; arrowheads, mitochondria showed damaged cristae with “onion-like” structure. Mitochondrial cristae in P525L-mutant FUS expressing cells were significantly more frequently disrupted than the control and Wt-FUS groups, with quantification shown in panel C. More than 50 mitochondria were quantified in each group, analyzed using Chi-square test (***:p<0.0001).(D) Quantification of mitochondrial size using Image J. Mitochondria in Wt- or P525L-mutant FUS expressing cells were significantly smaller than the control group. At least 50 mitochondria were quantified in each group, analyzed using one-way ANOVA with Bonferronipost test (***: p<0.0001). (E) Mitochondrion-associated FUS immunostaining signals were significantly increased in Wt or P525L FUS expressing cells as compared with the Ctr. At least 60 mitochondria were quantified in each group, analyzed using one-way ANOVA with Bonferronipost test (**: p<0.01; ***: p<0.0001).
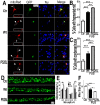
Fig 4. Increased expression of Wt- or P525L-mutant FUS induced a decrease in mitochondrial membrane potential and an increase in production of mitochondrial superoxide.
(A) Confocal images of live TMRM-stained HEK293 cells following transfection with corresponding plasmids: the GFP control, Wt- or P525L-mutant FUS tagged with GFP. All images were acquired in Z-stacks and projected as single images. Cells were stained with TMRM (a mitochondrial membrane potentiometric dye) and Hoechst 33342 before imaging.(B, C)FACS analyses of GFP-positive cells from experiments in panel A with quantification of TMRM signal intensity. Cells expressing either Wt- or P525L-mutant FUS showed decreased TMRM intensity as compared with the control group. The data were analyzed using one-way ANOVA (representing 4 independent experiments; *: p< 0.05, ***: p< 0.001). (D) Confocal images of mitoSox Red-stained HEK293 cells 24-hrs following transfection as described in panel A and Hoechst 33342 staining. (E, F) FACS analyses and quantification of mitoSOX-Red stained cells shown in panel D. Cells expressing P525L-mutant FUS showed significantly increased mitoSOX staining signals as compared with cells expressing the control or Wt-FUS protein. Data from 3 independent experiments were analyzed using one-way ANOVA (**:p< 0.01).
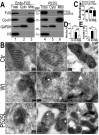
Fig 5. Increased FUS protein levels and mitochondrial damage detected in 3 independent FTLD-FUS brain samples.
(A, B)Fronto-cortical tissues of postmortem brain samples from three patients diagnosed with FTLD-FUS (#A, B and C) or 6 control cases (Ctr; #C1-#C6) were lysed in 1%SDS-containing hot lysis buffer (see Methods) and used for Western blotting with anti-FUS or HSP60 antibodies. Beta-actin was used as an internal control. In panel B is quantification of data in panel A, representing 3 independent samples (*: P<0.05;**: p<0.01; ANOVA with student t test). (C) IEM of FTLD-FUS and control brain samples. Cryosections of corresponding brain samples were immuno-labeled with specific anti-FUS antibody and secondary anti-murine IgG conjugated to 10-nm gold particles. Mitochondria were labeled with rabbit-anti-Tomm20 and anti-rabbit IgG conjugated to 25-nm gold particles. Arrows mark FUS-immunostaining signals labeled with 10-nm immuno-gold particles; arrowheads, mitochondria showing damaged cristae.(D) Mitochondrion-associated FUS immunostaining signals were significantly increased in FTLD-FUS cases as compared with the controls(***: p<0.0001). (E) Mitochondrial cristae showed significant damage in FTLD-FUS samples as compared with the control samples. At least 30 mitochondria were quantified in each group and analyzed using Chi-square test (**:p<0.01).Data represent three independent experiments.
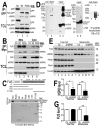
Fig 6. HSP60 interacts with FUS, mediating FUS mitochondrial localization.
(A) FUS-HSP60 interaction was detected by co-immunoprecipitation assay. Western blotting (WB) was performed using corresponding specific antibodies following immunoprecipitation of cell lysates with anti-GFP.(B)FUS-HSP60 interaction was detected by co-immunoprecipitation assay in both mitochondrial and cytosolic fractions. WB was performed using corresponding specific antibodies following immunoprecipitation of mitochondrial or cytosolic fractions with an anti-Myc monoclonal antibody. It should be noted that the protein concentrations of purified mitochondrial fraction relative to the cytosolic fraction is 10 to 1 and that approximately 5% of total FUS protein is localized to mitochondria.(C) GST pull-down experiments using purified FUS and HSP60 proteins indicate that the full-length (FL) Wt- or P525L-mutant FUS or the carboxyl terminal fragment (aa371-526) of FUS protein interacts with HSP60. GST pull-down proteins were analyzed by WB using the anti-HSP60 antibody. The lines below the FUS protein domain diagram depict different truncation mutants of FUS as GST-tagged proteins, with the corresponding amino acid residues shown. The lower panel of the gel image shows Coommassie blue staining of corresponding purified GST-FUS fusion proteins used. (D) Detection of direct FUS-HSP60 interaction by cross-linking coupled immunoprecipitation in live cells expressing the P525L-mutant FUS as a 6XHis-Myc tagged protein. The 130kDa cross-linked FUS-HSP60 species was detected by WB using the anti-HSP60 antibody in the pull-down proteins (“pull down”) following tandem-affinity purification using His-resins and anti-Myc beads (lane 2) or using anti-FUS in the “pull down” proteins following further immunoaffinity purification by anti-HSP60 (lane 8). Briefly, following 16-hour tetracycline induction of FUS expression, the control orP525L-mutant FUS expressing cells were treated with a cross-linking agent, formaldehyde (1%) for 10 min. FUS proteins were tandem-affinity purified using His-resin and anti-myc antibody and then analyzed by WB. A band was detected by anti-HSP60 antibody at 130-kDa in the pull-down fraction as marked by the arrow, whereas multiple bands including 60- and 130-kDa were detected in the input cross-linked cell lysates (input). HSP60 and the tagged FUS were detected at a size of 60 and 70 kDa, respectively, consistent with the prediction that the HSP60 containing 130kDa band consists of HSP60 and 6XHis-Myc tagged FUS. (E) Down-regulating HSP60 leads to a reduction in FUS mitochondrial localization. HEK293 cells were transfected with the control or HSP60-specific siRNAs and harvested for mitochondrial purification 72-hr post-transfection. The mitochondrial purity was confirmed by the enrichment of mitochondrial TOM20 and the absence of cytoplasmic protein such as RhoA or nuclear protein PCNA. Triplicates of the experiments are shown. (F) Quantification of the HSP60 levels in the total cell extracts and in the mitochondrial fractions. (G) Quantification of FUS levels in total cell extracts and the mitochondrial fractions. All data represent 3 independent experiments with statistical analyses using one-way ANOVA with Bonferronipost-test (*: p<0.05; **: p<0.01).
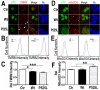
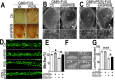
Fig 7. Down-regulating fly HSP60B expression partially rescues neurodegeneration phenotypes in FUS transgenic flies.
(A)FUS genetically interacts with HSP60B. Co-expressing siHSP60B with FUS in transgenic flies significantly rescued FUS-induced retinal defects as compared with the control. (B, C)Scanning electron microscopic images of fly eyes expressing FUS together with the control siRNA or siHSP60B, with the lower panels showing higher magnification images of the corresponding groups. Flies co-expressing siHSP60B and FUS showed more well-organized bristles and reduced ommatidial fusion as compared with FUS transgenic flies expressing the control siRNA. Fly genotypes: Ctr: GMR-Gal4/UAS-Wt-FUS-RFP or GMR-Gal4/UAS-P525L-FUS-RFP; siHSP60B: GMR-Gal4/UAS-Wt-FUS-RFP/UAS-siHSP60B or GMR-Gal4/UAS-P525L-FUS-RFP/UAS-siHSP60B. (D)Confocal microscopic images of mitochondria in motor neuron axons in the larval A3 abdominal segments(Z-stacks projected into single images). Fly genotypes, Ctr: D42-Gal4/UAS-mitoGFP/UAS-RFP; siHSP60B: D42-Gal4/UAS-mitoGFP/UAS-siHSP60B; P525L: D42-Gal4/UAS-mitoGFP/UAS-P525L-FUS-RFP; P525L-FUS+siHSP60B: D42-Gal4/UAS-mitoGFP/UAS-P525L-FUS-RFP/UAS-siHSP60B. (E) RNAi mediated down-regulation of HSP60B in P525L-mutant FUS expressing flies rescued mitochondrial fragmentation phenotype, as shown by quantification of mitochondrial lengths in MN axons. The 3rd instar larvae expressing P525L-mutant FUS showed significantly smaller mitochondria as compared with the control. Ten larvae were measured in each group. (F, G)Knocking-down HSP60B in flies expressing P525L-mutant FUS in MNs significantly rescued their larval locomotive defects. The tail paralysis phenotype was examined in third instar larvae, as measured by the loss of ability for larvae to anchor their tails on the agar surface when crawling {as previously published [17]}. Fly genotypes, Ctr: OK371-Gal4/UAS-RFP; siHSP60B: OK371-Gal4/UAS-siHSP60B; P525L: OK371-Gal4/UAS-P525L-FUS-RFP; P525L-FUS+siHSP60B: OK371-Gal4/UAS-P525L-FUS-RFP/UAS-siHSP60B. All data were analyzed using one-way ANOVA with Bonferroni post-test (*: p<0.05; ***: p<0.001).
Fig 8. Down-regulating HSP60B in fly photoreceptors partially rescues the retinal degeneration phenotype of FUS transgenic mice as shown by transmission electron microscopy (TEM).
Electron microscopic images of fly retinas at day 3 are shown, together with mitochondria and nuclei (at higher magnifications). Rh: rhabdomere (marked by arrows); Mito: mitochondria; Nu: nuclei. The retinas in the control flies display normal rhabdomere organization with seven photoreceptor cells and healthy mitochondria at day 3, but the flies expressing either Wt- or P525L-mutant FUS show disrupted rhabdomere organization and smaller mitochondria. Knocking-down HSP60B expression by specific siRNA partially rescued these defects in flies expressing Wt- or P525L-mutant FUS.
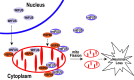
Fig 9. A working model for FUS-induced neurotoxicity.
Either an aberrant increase in the expression of Wt-FUS (WtFUS) or FUS mutations that enhance the cytoplasmic redistribution of the mutant FUS protein (mtFUS) leads to abnormal accumulation of cytoplasmic FUS and increased translocation to mitochondria, resulting in excessive mitochondrial fission and damage that eventually culminate in neuronal death.
Similar articles
- FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models.
Deng J, Wang P, Chen X, Cheng H, Liu J, Fushimi K, Zhu L, Wu JY. Deng J, et al. Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):E9678-E9686. doi: 10.1073/pnas.1806655115. Epub 2018 Sep 24. Proc Natl Acad Sci U S A. 2018. PMID: 30249657 Free PMC article. - PINK1 and Parkin are genetic modifiers for FUS-induced neurodegeneration.
Chen Y, Deng J, Wang P, Yang M, Chen X, Zhu L, Liu J, Lu B, Shen Y, Fushimi K, Xu Q, Wu JY. Chen Y, et al. Hum Mol Genet. 2016 Dec 1;25(23):5059-5068. doi: 10.1093/hmg/ddw310. Hum Mol Genet. 2016. PMID: 27794540 Free PMC article. - Self-assembly of FUS through its low-complexity domain contributes to neurodegeneration.
Matsumoto T, Matsukawa K, Watanabe N, Kishino Y, Kunugi H, Ihara R, Wakabayashi T, Hashimoto T, Iwatsubo T. Matsumoto T, et al. Hum Mol Genet. 2018 Apr 15;27(8):1353-1365. doi: 10.1093/hmg/ddy046. Hum Mol Genet. 2018. PMID: 29425337 - Role of Mitochondrial Stress Protein HSP60 in Diabetes-Induced Neuroinflammation.
Liyanagamage DSNK, Martinus RD. Liyanagamage DSNK, et al. Mediators Inflamm. 2020 Apr 17;2020:8073516. doi: 10.1155/2020/8073516. eCollection 2020. Mediators Inflamm. 2020. PMID: 32410865 Free PMC article. Review. - FUS-related proteinopathies: lessons from animal models.
Lanson NA Jr, Pandey UB. Lanson NA Jr, et al. Brain Res. 2012 Jun 26;1462:44-60. doi: 10.1016/j.brainres.2012.01.039. Epub 2012 Jan 25. Brain Res. 2012. PMID: 22342159 Review.
Cited by
- Neurodegenerative disorders, metabolic icebergs, and mitohormesis.
Phillips MCL, Picard M. Phillips MCL, et al. Transl Neurodegener. 2024 Sep 6;13(1):46. doi: 10.1186/s40035-024-00435-8. Transl Neurodegener. 2024. PMID: 39242576 Free PMC article. Review. - Axonopathy Underlying Amyotrophic Lateral Sclerosis: Unraveling Complex Pathways and Therapeutic Insights.
Luan T, Li Q, Huang Z, Feng Y, Xu D, Zhou Y, Hu Y, Wang T. Luan T, et al. Neurosci Bull. 2024 Aug 4. doi: 10.1007/s12264-024-01267-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39097850 Review. - The Role of Methylation in Ferroptosis.
Xie Y, Xie J, Li L. Xie Y, et al. J Cardiovasc Transl Res. 2024 Jul 29. doi: 10.1007/s12265-024-10539-1. Online ahead of print. J Cardiovasc Transl Res. 2024. PMID: 39075241 Review. - Metabolic regulation of misfolded protein import into mitochondria.
Wang Y, Ruan L, Zhu J, Zhang X, Chang AC, Tomaszewski A, Li R. Wang Y, et al. Elife. 2024 Jun 20;12:RP87518. doi: 10.7554/eLife.87518. Elife. 2024. PMID: 38900507 Free PMC article. - Aberrant protein aggregation in amyotrophic lateral sclerosis.
Wang H, Zeng R. Wang H, et al. J Neurol. 2024 Aug;271(8):4826-4851. doi: 10.1007/s00415-024-12485-z. Epub 2024 Jun 13. J Neurol. 2024. PMID: 38869826 Review.
References
- Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, et al. (1993) Mild ALS in Japan associated with novel SOD mutation. Nat Genet 5: 323–324. - PubMed
- Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364: 362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- R01AG033004/AG/NIA NIH HHS/United States
- R56NS074763/NS/NINDS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- R01 AG033004/AG/NIA NIH HHS/United States
- U54 CA151880/CA/NCI NIH HHS/United States
- P30 AG13854/AG/NIA NIH HHS/United States
- R56 NS074763/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous