Differential Susceptibilities of Human Lung Primary Cells to H1N1 Influenza Viruses - PubMed (original) (raw)
Comparative Study
. 2015 Dec;89(23):11935-44.
doi: 10.1128/JVI.01792-15. Epub 2015 Sep 16.
Affiliations
- PMID: 26378172
- PMCID: PMC4645340
- DOI: 10.1128/JVI.01792-15
Comparative Study
Differential Susceptibilities of Human Lung Primary Cells to H1N1 Influenza Viruses
Emily Travanty et al. J Virol. 2015 Dec.
Abstract
Human alveolar epithelial cells (AECs) and alveolar macrophages (AMs) are the first lines of lung defense. Here, we report that AECs are the direct targets for H1N1 viruses that have circulated since the 2009 pandemic (H1N1pdm09). AMs are less susceptible to H1N1pdm09 virus, but they produce significantly more inflammatory cytokines than AECs from the same donor. AECs form an intact epithelial barrier that is destroyed by H1N1pdm09 infection. However, there is significant variation in the cellular permissiveness to H1N1pdm09 infection among different donors. AECs from obese donors appear to be more susceptible to H1N1pdm09 infection, whereas gender, smoking history, and age do not appear to affect AEC susceptibility. There is also a difference in response to different strains of H1N1pdm09 viruses. Compared to A/California04/09 (CA04), A/New York/1682/09 (NY1682) is more infectious and causes more epithelial barrier injury, although it stimulates less cytokine production. We further determined that a single amino acid residue substitution in NY1682 hemagglutinin is responsible for the difference in infectivity. In conclusion, this is the first study of host susceptibility of human lung primary cells and the integrity of the alveolar epithelial barrier to influenza. Further elucidation of the mechanism of increased susceptibility of AECs from obese subjects may facilitate the development of novel protection strategies against influenza virus infection.
Importance: Disease susceptibility of influenza is determined by host and viral factors. Human alveolar epithelial cells (AECs) form the key line of lung defenses against pathogens. Using primary AECs from different donors, we provided cellular level evidence that obesity might be a risk factor for increased susceptibility to influenza. We also compared the infections of two closely related 2009 pandemic H1N1 strains in AECs from the same donor and identified a key viral factor that affected host susceptibility, the dominance of which may be correlated with disease epidemiology. In addition, primary human AECs can serve as a convenient and powerful model to investigate the mechanism of influenza-induced lung injury and determine the effect of genetic and epigenetic factors on host susceptibility to pandemic influenza virus infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
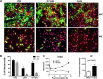
Infection of H1N1 viruses with primary human AECs and AMs from the same donor. (A and B) Infection of AECs and AMs. AECs and AMs from the same donor were cultured on glass coverslips and infected with H1N1 viruses at an MOI of 1. At 24 h postinoculation, cells were fixed with methanol and doubly stained for viral nucleoprotein (NP) (green) and cell markers (red): cytokeratin for AEC and CD68 for AM. Nuclei were counterstained with DAPI (blue). (A) Representative images of infected cells; (B) quantification of infections. Significant differences between AEC and AM groups are indicated as follows: *, P < 0.05, and **, P < 0.01; n = 3. (C) H1N1 virus causes barrier injury in AECs. AECs were cultured on the inserts and infected with H1N1pdm09 virus NY1682 at an MOI of 1. TEER was measured at different time points. Data represent results from one of four donors. Paracellular permeability (PP) was measured at 72 h postinfection; n = 4.
FIG 2
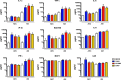
Cytokine and chemokine responses in human AECs and AMs infected with H1N1 viruses. Human AECs and AMs from the same donors were infected with same amount of H1N1 viruses PR8, CA04, NY1682, and CA07 (MOI = 1), and culture supernatant was collected for detection of the cytokine and chemokine responses by multiplex assay and ELISA by following the manufacturer's instructions. Significant differences from the mock control are indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001; n = 3.
FIG 3
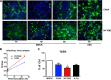
Variation in human AEC susceptibility to H1N1pdm09 viruses. AECs from the same donor were infected with CA04 or NY1682 at an MOI of 1. At 24 hpi, cells were fixed with methanol and immunostained with anti-NP. Data represent percentages of positive infected cells for each virus. Each spot indicates one donor. (A) Differential susceptibilities to H1N1pdm09 viruses; (B) effects of age, gender, smoking, and obesity on AEC susceptibility to CA04. BMI, body mass index; NS, nonsmoker; Ex-S, ex-smoker; S, smoker.
FIG 4
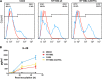
Comparison of viral entry, replication, and effect on AEC barrier between NY1682 and CA04 infections. (A) Viral entry in MDCK cells and human AECs. MDCK cells and human AECs were inoculated with the same amount of CA04 or NY1682 at 4°C for 2 h and washed with DMEM before incubation at 37°C. Cells were harvested at designated time points for immunostaining with anti-influenza virus NP (green) for MDCK cells or antihemagglutinin (anti-HA) (green) for AECs; nuclei were counterstained with DAPI (blue). The experiments were repeated twice. (B and C) Viral replication and effect on human AEC barrier. AECs were infected with and without CA04 or NY1682 (MOI = 1) or treated with recombinant human IL-1β (10 ng/ml) as a positive control. (B) Culture supernatant was collected for evaluation of infectious virus release by plaque assay as described previously (6). **, P < 0.01 between two groups. (C) TEER was measured at 24 h posttreatment. Significant differences from the mock control are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 4. # indicates a significant difference from the CA04 group.
FIG 5
NY1682-HA-S100P/D293N/V338I (rNY1682-CA07HA) behaves similarly to NY1682 in the ability to infect human AECs. Human AECs were infected with recombinant NY1682 carrying HA-S100P/D293N/V338I (CA07HA), wild-type NY1682, and CA04. Percentages of infection were compared by flow cytometry, and induction of IL-29 release was measured by ELISA. (A) Infectivity; (B) IL-29 release. Data represent results from one of three donors.
FIG 6
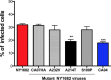
Replacement of Ala with Thr at position 214 of NY1682-HA reduces the infectivity of NY1682. AECs infected with wild-type NY1682, CA04, or recombinant NY1682 viruses carrying HA-S100P/D293N/V338I (CA07HA), -A214T, -Al238V, or -S100P were fixed with 4% paraformaldehyde and immunostained with FITC-labeled anti-influenza virus NP. Positive infected cells were detected by flow cytometry, and percentages of infection were compared among different viruses; n = 5. **, P < 0.01; ***, P < 0.001.
FIG 7
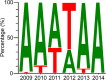
Proportion of H1N1pdm09 viruses that contain an alanine or threonine at HA residue 214 from 2009 to 2014. All pH1N1 HA sequences in GenBank were downloaded and grouped by the years of sample collection. For each year, the HA protein sequences were aligned and all amino acids at residue 214 were illustrated based on their relative frequencies using the WebLogo application (49).
Similar articles
- Serial Section Array Scanning Electron Microscopy Analysis of Cells from Lung Autopsy Specimens following Fatal A/H1N1 2009 Pandemic Influenza Virus Infection.
Kataoka M, Ishida K, Ogasawara K, Nozaki T, Satoh YI, Sata T, Sato Y, Hasegawa H, Nakajima N. Kataoka M, et al. J Virol. 2019 Sep 12;93(19):e00644-19. doi: 10.1128/JVI.00644-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292247 Free PMC article. - Infection of lung epithelial cells with pandemic 2009 A(H1N1) influenza viruses reveals isolate-specific differences in infectivity and host cellular responses.
Patel JR, Vora KP, Tripathi S, Zeng H, Tumpey TM, Katz JM, Sambhara S, Gangappa S. Patel JR, et al. Viral Immunol. 2011 Apr;24(2):89-99. doi: 10.1089/vim.2010.0122. Viral Immunol. 2011. PMID: 21449719 - Swine Influenza Virus PA and Neuraminidase Gene Reassortment into Human H1N1 Influenza Virus Is Associated with an Altered Pathogenic Phenotype Linked to Increased MIP-2 Expression.
Dlugolenski D, Jones L, Howerth E, Wentworth D, Tompkins SM, Tripp RA. Dlugolenski D, et al. J Virol. 2015 May;89(10):5651-67. doi: 10.1128/JVI.00087-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762737 Free PMC article. - Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses.
Guarner J, Falcón-Escobedo R. Guarner J, et al. Arch Med Res. 2009 Nov;40(8):655-61. doi: 10.1016/j.arcmed.2009.10.001. Epub 2010 Jan 6. Arch Med Res. 2009. PMID: 20304252 Review.
Cited by
- Respiratory Mononuclear Phagocytes in Human Influenza A Virus Infection: Their Role in Immune Protection and As Targets of the Virus.
Vangeti S, Yu M, Smed-Sörensen A. Vangeti S, et al. Front Immunol. 2018 Jul 3;9:1521. doi: 10.3389/fimmu.2018.01521. eCollection 2018. Front Immunol. 2018. PMID: 30018617 Free PMC article. Review. - Impact of Body Mass Index on COVID-19-Related In-Hospital Outcomes and Mortality.
Ullah W, Roomi S, Nadeem N, Saeed R, Tariq S, Ellithi M, Haq S, Arslan A, Madara J, Boigon M, Haas DC, Fischman DL. Ullah W, et al. J Clin Med Res. 2021 Apr;13(4):230-236. doi: 10.14740/jocmr4239. Epub 2021 Apr 27. J Clin Med Res. 2021. PMID: 34007361 Free PMC article. - Alternative Experimental Models for Studying Influenza Proteins, Host-Virus Interactions and Anti-Influenza Drugs.
Chua SCJH, Tan HQ, Engelberg D, Lim LHK. Chua SCJH, et al. Pharmaceuticals (Basel). 2019 Sep 30;12(4):147. doi: 10.3390/ph12040147. Pharmaceuticals (Basel). 2019. PMID: 31575020 Free PMC article. Review. - Impact of e-cigarette aerosol on primary human alveolar epithelial type 2 cells.
Wick KD, Fang X, Maishan M, Matsumoto S, Spottiswoode N, Sarma A, Simoneau C, Khakoo M, Langelier C, Calfee CS, Gotts JE, Matthay MA. Wick KD, et al. Am J Physiol Lung Cell Mol Physiol. 2022 Aug 1;323(2):L152-L164. doi: 10.1152/ajplung.00503.2021. Epub 2022 Jun 7. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35670478 Free PMC article. - AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality.
Zhang H, Luo J, Alcorn JF, Chen K, Fan S, Pilewski J, Liu A, Chen W, Kolls JK, Wang J. Zhang H, et al. J Immunol. 2017 Jun 1;198(11):4383-4393. doi: 10.4049/jimmunol.1600714. Epub 2017 Apr 19. J Immunol. 2017. PMID: 28424239 Free PMC article.
References
- Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR. 2010. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 177:166–175. doi:10.2353/ajpath.2010.100115. - DOI - PMC - PubMed
- Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, Alves VA, Malheiros DM, Auler JO Jr, Ferreira AF, Borsato MR, Bezerra SM, Gutierrez PS, Caldini ET, Pasqualucci CA, Dolhnikoff M, Saldiva PH. 2010. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 181:72–79. doi:10.1164/rccm.200909-1420OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R03 AI101953/AI/NIAID NIH HHS/United States
- R01HL113655/HL/NHLBI NIH HHS/United States
- U01AI082982/AI/NIAID NIH HHS/United States
- R01 HL091938/HL/NHLBI NIH HHS/United States
- R03AI101953/AI/NIAID NIH HHS/United States
- R01 HL107380/HL/NHLBI NIH HHS/United States
- R01 HL113655/HL/NHLBI NIH HHS/United States
- R01 HL125128/HL/NHLBI NIH HHS/United States
- U01 AI082982/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical