YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers - PubMed (original) (raw)
. 2015 Oct 15;60(2):328-37.
doi: 10.1016/j.molcel.2015.09.001. Epub 2015 Oct 1.
Matteo Carrara 2, Wei-Chien Yuan 1, Christian Valdes-Quezada 3, Basanta Gurung 4, Brian Pepe-Mooney 5, Tinghu Zhang 6, Geert Geeven 3, Nathanael S Gray 6, Wouter de Laat 3, Raffaele A Calogero 2, Fernando D Camargo 7
Affiliations
- PMID: 26439301
- PMCID: PMC4624327
- DOI: 10.1016/j.molcel.2015.09.001
YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers
Giorgio G Galli et al. Mol Cell. 2015.
Abstract
The Hippo/YAP signaling pathway is a crucial regulator of tissue growth, stem cell activity, and tumorigenesis. However, the mechanism by which YAP controls transcription remains to be fully elucidated. Here, we utilize global chromatin occupancy analyses to demonstrate that robust YAP binding is restricted to a relatively small number of distal regulatory elements in the genome. YAP occupancy defines a subset of enhancers and superenhancers with the highest transcriptional outputs. YAP modulates transcription from these elements predominantly by regulating promoter-proximal polymerase II (Pol II) pause release. Mechanistically, YAP interacts and recruits the Mediator complex to enhancers, allowing the recruitment of the CDK9 elongating kinase. Genetic and chemical perturbation experiments demonstrate the requirement for Mediator and CDK9 in YAP-driven phenotypes of overgrowth and tumorigenesis. Our results here uncover the molecular mechanisms employed by YAP to exert its growth and oncogenic functions, and suggest strategies for intervention.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
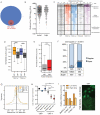
Figure 1. YAP binds to potent enhancers
A) Venn diagram of the common TEAD1/4 and YAP peaks in HuCCT1 cells. B) Scatter plot of the distance distribution of YAP peaks from the closest TSS in the indicated cell lines. C) Heatmap for the ChIP-seq signal of the indicated antibodies +/−2Kb from the center of YAP peaks. Clustering results from K-means method. D) Box plots of the normalized counts of H3K27ac and H3K4me1 signal at YAP+ enhancer peaks or YAP− enhancers. *** p<0.0001. E) Boxplot indicating expression levels of genes associated to YAP+ or YAP− enhancers following RNAseq in HUCCT1 cells. F) Histogram indicating the fraction of YAP− or YAP+ enhancers catalogued as regular o super- enhancers in HuCCT1 cells. G) Line plots depicting H3K27Ac and H3K3me1 average signal in YAP+ enhancers upon siYT treatment. H) Log2 changes in gene expression between siControl / siYT treated cells for genes associated with different genomic elements 48h post transfection. Error bars represent 95% CI. I) Left panel, ChIP-qPCR for H3K27ac in selected YREs in cells at low and high density. Error bars = SD. Right panel, immunofluorescence for YAP in these conditions. See also Figure S1.
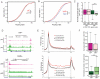
Figure 2. YAP controls RNA Polymerase II promoter pause-release
A–B) Line graph representing the pausing index (PI) for YAP positive genes upon YAP/TAZ silencing (siYT) (A); and YAP+ enhancer genes vs. YAP− (B). C) Box plot representing the fold change of RNA PolII pS5 at the promoter (pink shades) or pS2 in the gene body (green shades) in YAP+ and YAP− enhancer genes upon siYT treatment. D) ChIP-seq tracks representing signal for RNA PolII pS5 or pS2 around a representative YAP− gene and a YAP+ gene. E) Metagene profile of RNAPolII pS5 (upper) and pS2 (lower) for YAP+ and YAP− super-enhancer genes in cells treated with siC and siYT. F) Box plot showing the occupancy of PolII pS5 at the promoter (pink shades) or pS2 in the gene body (green shades) in YAP+ and YAP− enhancer genes. See also Figure S2.
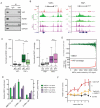
Figure 3. YAP recruits Mediator to regulate transcription
A) Validation co-IP between YAP-TEAD1 and MED12. B) ChIP-seq tracks of a YAP+ and YAP− locus for YAP, MED1 and SMC1 in HuCCT1 cells treated with siC or siYT. C) Boxplot representing normalized MED1 (left) or SMC1 (right) coverage in YAP+ or YAP− enhancers in siC- or siYT-treated cells. D) Scatter plot correlating YAP occupancy to loss of MED1 signal upon YAP/TAZ silencing. On the X-axis MED1 peaks are ranked according to YAP signal. Y-axis in the top panel represents MED1 signal changes between siC and siYT cells. Y-axis in the lower panel represents the counts of YAP signal in each MED1 peak. E) ChIP-qPCR for MED1 and SMC1 at selected YREs in H69 cells at low and high density. Error bars = SD. F) Tumor volume of xenograft HuCCT1 bearing TetOYAP and/or shMED1 constructs relative to volume measured before the administration of Doxycycline (n= 5 mice, error bars = SEM). See also Figure S3.
Figure 4. YAP-Mediator regulates transcriptional elongation via CDK9 recruitment
A) Normalized CDK9 coverage around the promoter of YAP negative- and YRE-associated genes. Error bars = 95% CI B) Fold change of CDK9 occupancy upon siYT treatment around the promoter of YAP negative- and YRE-associated genes. Error bars = 95% CI C) Log2 (FC) of expression of YAP− vs. YAP+ genes in HuCCT1 cells treated with CDK9 inhibitors Flavopiridol and NVP-2. Error bars = 95% CI. D) Liver/Body weight ratio of mice overexpressing YAP for 1 week treated with vehicle or Flavopiridol. E) Model for YAP/Mediator-driven transcriptional elongation in active Hippo signaling (left) or inactive Hippo signaling (right). See also Figure S4.
Comment in
- YAP controls transcriptional elongation through CKD9 recruitment for proximal pause release: "Hippo-thetical", new therapeutic targets?
Kyritsi F, Price DK, Figg WD. Kyritsi F, et al. Cancer Biol Ther. 2016 Jun 2;17(6):592-4. doi: 10.1080/15384047.2016.1178883. Epub 2016 May 5. Cancer Biol Ther. 2016. PMID: 27149279 Free PMC article.
Similar articles
- YAP and TAZ Heterogeneity in Primary Liver Cancer: An Analysis of Its Prognostic and Diagnostic Role.
Van Haele M, Moya IM, Karaman R, Rens G, Snoeck J, Govaere O, Nevens F, Verslype C, Topal B, Monbaliu D, Halder G, Roskams T. Van Haele M, et al. Int J Mol Sci. 2019 Feb 1;20(3):638. doi: 10.3390/ijms20030638. Int J Mol Sci. 2019. PMID: 30717258 Free PMC article. - YAP/TAZ Initiates Gastric Tumorigenesis via Upregulation of MYC.
Choi W, Kim J, Park J, Lee DH, Hwang D, Kim JH, Ashktorab H, Smoot D, Kim SY, Choi C, Koh GY, Lim DS. Choi W, et al. Cancer Res. 2018 Jun 15;78(12):3306-3320. doi: 10.1158/0008-5472.CAN-17-3487. Epub 2018 Apr 18. Cancer Res. 2018. PMID: 29669762 - A Hippo and Fibroblast Growth Factor Receptor Autocrine Pathway in Cholangiocarcinoma.
Ilyas SI, Yamada D, Hirsova P, Bronk SF, Werneburg NW, Krishnan A, Salim W, Zhang L, Trushina E, Truty MJ, Gores GJ. Ilyas SI, et al. J Biol Chem. 2016 Apr 8;291(15):8031-47. doi: 10.1074/jbc.M115.698472. Epub 2016 Jan 29. J Biol Chem. 2016. PMID: 26826125 Free PMC article. - Targeting Hippo/YAP in intrahepatic cholangiocarcinoma: Promising molecules in cancer therapy.
Ma X, Zhou Y, Li R, Ding X, Li D, Pan T, Zhang F, Li W. Ma X, et al. Mol Carcinog. 2024 Oct;63(10):1866-1873. doi: 10.1002/mc.23791. Epub 2024 Aug 2. Mol Carcinog. 2024. PMID: 39092765 Review. - YAP/TAZ: An epitome of tumorigenesis.
Mukherjee S, Warden EA, Zhang J. Mukherjee S, et al. Cancer Lett. 2025 Aug 10;625:217806. doi: 10.1016/j.canlet.2025.217806. Epub 2025 May 15. Cancer Lett. 2025. PMID: 40381686 Review.
Cited by
- Induction of the TEAD Coactivator VGLL1 by Estrogen Receptor-Targeted Therapy Drives Resistance in Breast Cancer.
Gemma C, Lai CF, Singh AK, Belfiore A, Portman N, Milioli HZ, Periyasamy M, Raafat S, Nicholls AJ, Davies CM, Patel NR, Simmons GM, Fan H, Nguyen VTM, Magnani L, Rakha E, Martin LA, Lim E, Coombes RC, Pruneri G, Buluwela L, Ali S. Gemma C, et al. Cancer Res. 2024 Dec 16;84(24):4283-4297. doi: 10.1158/0008-5472.CAN-24-0013. Cancer Res. 2024. PMID: 39356622 Free PMC article. - Mitochondrial stress induces AREG expression and epigenomic remodeling through c-JUN and YAP-mediated enhancer activation.
Hino Y, Nagaoka K, Oki S, Etoh K, Hino S, Nakao M. Hino Y, et al. Nucleic Acids Res. 2022 Sep 23;50(17):9765-9779. doi: 10.1093/nar/gkac735. Nucleic Acids Res. 2022. PMID: 36095121 Free PMC article. - YAP controls transcriptional elongation through CKD9 recruitment for proximal pause release: "Hippo-thetical", new therapeutic targets?
Kyritsi F, Price DK, Figg WD. Kyritsi F, et al. Cancer Biol Ther. 2016 Jun 2;17(6):592-4. doi: 10.1080/15384047.2016.1178883. Epub 2016 May 5. Cancer Biol Ther. 2016. PMID: 27149279 Free PMC article. - Genetic variants in Hippo signalling pathway-related genes affect the risk of colorectal cancer.
Shen H, Meng Y, Hu T, Li S, Du M, Xin J, Gu D, Wang M, Fu Z. Shen H, et al. Arch Toxicol. 2021 Jan;95(1):271-281. doi: 10.1007/s00204-020-02910-3. Epub 2020 Oct 3. Arch Toxicol. 2021. PMID: 33011827 - A mutational hotspot in AMOTL1 defines a new syndrome of orofacial clefting, cardiac anomalies, and tall stature.
Strong A, Rao S, von Hardenberg S, Li D, Cox LL, Lee PC, Zhang LQ, Awotoye W, Diamond T, Gold J, Gooch C, Gowans LJJ, Hakonarson H, Hing A, Loomes K, Martin N, Marazita ML, Mononen T, Piccoli D, Pfundt R, Raskin S, Scherer SW, Sobriera N, Vaccaro C, Wang X, Watson D, Weksberg R, Bhoj E, Murray JC, Lidral AC, Butali A, Buckley MF, Roscioli T, Koolen DA, Seaver LH, Prows CA, Stottmann RW, Cox TC. Strong A, et al. Am J Med Genet A. 2023 May;191(5):1227-1239. doi: 10.1002/ajmg.a.63130. Epub 2023 Feb 7. Am J Med Genet A. 2023. PMID: 36751037 Free PMC article.
References
- Adelman K, Kennedy MA, Nechaev S, Gilchrist DA, Muse GW, Chinenov Y, Rogatsky I. Immediate mediators of the inflammatory response are poised for gene activation through RNA polymerase II stalling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18207–18212. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous