Risk of Bias in Reports of In Vivo Research: A Focus for Improvement - PubMed (original) (raw)
Comparative Study
. 2015 Oct 13;13(10):e1002273.
doi: 10.1371/journal.pbio.1002273. eCollection 2015 Oct.
Aaron Lawson McLean 1, Aikaterini Kyriakopoulou 1, Stylianos Serghiou 1, Arno de Wilde 2, Nicki Sherratt 1, Theo Hirst 1, Rachel Hemblade 1, Zsanett Bahor 1, Cristina Nunes-Fonseca 1, Aparna Potluru 1, Andrew Thomson 1, Julija Baginskaite, Kieren Egan 1, Hanna Vesterinen 1, Gillian L Currie 1, Leonid Churilov 3, David W Howells 4, Emily S Sena 5
Affiliations
- PMID: 26460723
- PMCID: PMC4603955
- DOI: 10.1371/journal.pbio.1002273
Comparative Study
Risk of Bias in Reports of In Vivo Research: A Focus for Improvement
Malcolm R Macleod et al. PLoS Biol. 2015.
Erratum in
- Correction: Risk of Bias in Reports of In Vivo Research: A Focus for Improvement.
Macleod MR, Lawson McLean A, Kyriakopoulou A, Serghiou S, de Wilde A, Sherratt N, Hirst T, Hemblade R, Bahor Z, Nunes-Fonseca C, Potluru A, Thomson A, Baginskaite J, Egan K, Vesterinen H, Currie GL, Churilov L, Howells DW, Sena ES. Macleod MR, et al. PLoS Biol. 2015 Nov 10;13(11):e1002301. doi: 10.1371/journal.pbio.1002301. eCollection 2015 Nov. PLoS Biol. 2015. PMID: 26556632 Free PMC article.
Abstract
The reliability of experimental findings depends on the rigour of experimental design. Here we show limited reporting of measures to reduce the risk of bias in a random sample of life sciences publications, significantly lower reporting of randomisation in work published in journals of high impact, and very limited reporting of measures to reduce the risk of bias in publications from leading United Kingdom institutions. Ascertainment of differences between institutions might serve both as a measure of research quality and as a tool for institutional efforts to improve research quality.
Conflict of interest statement
MRM is a member of the PLOS Medicine Editorial Board.
Figures
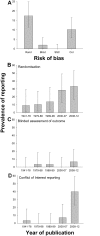
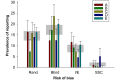
Fig 1. (A) Prevalence of reporting of randomisation, blinded assessment of outcome, sample size calculation, and conflict of interest in 146 publications describing in vivo research identified through random sampling from PubMed; change in prevalence of (B) randomisation, (C) blinded assessment of outcome, and (D) conflict of interest reporting in quintiles of year of publication.
Vertical error bars represent the 95% confidence intervals of the estimates (S1 Data).
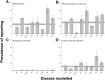
Fig 2. Prevalence of reporting of (A) randomisation, (B) blinded assessment of outcome, (C) sample size calculations, and (D) conflict of interest reporting in 2,671 publications describing the efficacy of interventions in animal models of Alzheimer’s disease (AD, n = 324 publications), focal cerebral ischaemia (FCI, 704), glioma (175), Huntington’s disease (HD, 113), intracerebral haemorrhage (ICH, 72), experimental autoimmune encephalomyelitis (EAE, 1029), myocardial infarction (MI, 69), and spinal cord injury (SCI, 185) identified in the context of systematic reviews.
Vertical error bars represent the 95% confidence intervals, and the horizontal grey bar represents the 95% confidence interval of the overall estimate (S2 Data).
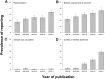
Fig 3. Change in prevalence of reporting of (A) randomisation, (B) blinded assessment of outcome, (C) sample size calculations, and (D) conflict of interest reporting in quintiles of year of publication for 2,671 publications describing the efficacy of interventions in animal models of eight different diseases identified in the context of systematic reviews.
Vertical error bars represent the 95% confidence intervals of the estimates (S3 Data).
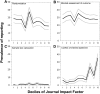
Fig 4. Prevalence of reporting of (A) randomisation, (B) blinded assessment of outcome, (C) sample size calculations, and (D) conflict of interest reporting by decile of journal impact factor in 2,671 publications describing the efficacy of interventions in animal models of eight different diseases identified in the context of systematic reviews.
Black lines indicate the median value in that decile, and grey lines indicate the 95% confidence limits derived from nonparametric median regression (S4 Data).
Fig 5. Prevalence of reporting of randomisation, blinded assessment of outcome, inclusion or exclusion criteria, and sample size calculation in 1,173 publications describing in vivo research published from five leading UK institutions (labelled A through E).
For each institution, the vertical error bars represent the 95% confidence intervals, and the horizontal grey bar represents the 95% confidence interval of the overall estimate for that risk-of-bias item (S5 Data).
Comment in
- Poor quality animal studies cause clinical trials to follow false leads.
Hawkes N. Hawkes N. BMJ. 2015 Oct 13;351:h5453. doi: 10.1136/bmj.h5453. BMJ. 2015. PMID: 26468237 No abstract available.
Similar articles
- Imaging mass spectrometry: a user’s guide to a new technique for biological and biomedical research. Preface.
McDonnell L, Andrén PE, Corthals GL. McDonnell L, et al. J Proteomics. 2012 Aug 30;75(16):4881-4882. doi: 10.1016/j.jprot.2012.07.033. J Proteomics. 2012. PMID: 22901460 No abstract available. - The FEBS Journal: hidden gems.
Breuer M. Breuer M. FEBS J. 2021 Dec;288(23):6586-6588. doi: 10.1111/febs.16087. Epub 2021 Jul 15. FEBS J. 2021. PMID: 34263998 - Poor quality animal studies cause clinical trials to follow false leads.
Hawkes N. Hawkes N. BMJ. 2015 Oct 13;351:h5453. doi: 10.1136/bmj.h5453. BMJ. 2015. PMID: 26468237 No abstract available. - China's Contribution to Anesthesiology Research: A 10-Year Survey of the Literature.
Xie G, Zhang K, Wood C, Hoeft A, Liu J, Fang X. Xie G, et al. Anesth Analg. 2016 May;122(5):1640-5. doi: 10.1213/ANE.0000000000001225. Anesth Analg. 2016. PMID: 27003916 Review. - [Fundamental studies in medico-biological sciences].
Kryzhanovskiĭ GN. Kryzhanovskiĭ GN. Vestn Ross Akad Med Nauk. 2006;(3):31-3. Vestn Ross Akad Med Nauk. 2006. PMID: 16734341 Review. Russian. No abstract available.
Cited by
- Disease coverage of human genome-wide association studies and pharmaceutical research and development.
Gordillo-Marañón M, Schmidt AF, Warwick A, Tomlinson C, Ytsma C, Engmann J, Torralbo A, Maclean R, Sofat R, Langenberg C, Shah AD, Denaxas S, Pirmohamed M, Hemingway H, Hingorani AD, Finan C. Gordillo-Marañón M, et al. Commun Med (Lond). 2024 Oct 8;4(1):195. doi: 10.1038/s43856-024-00625-5. Commun Med (Lond). 2024. PMID: 39379679 Free PMC article. - Estimating the replicability of highly cited clinical research (2004-2018).
da Costa GG, Neves K, Amaral O. da Costa GG, et al. PLoS One. 2024 Aug 7;19(8):e0307145. doi: 10.1371/journal.pone.0307145. eCollection 2024. PLoS One. 2024. PMID: 39110675 Free PMC article. - SinglePointRNA, an user-friendly application implementing single cell RNA-seq analysis software.
Puente-Santamaría L, Del Peso L. Puente-Santamaría L, et al. PLoS One. 2024 Jun 18;19(6):e0300567. doi: 10.1371/journal.pone.0300567. eCollection 2024. PLoS One. 2024. PMID: 38889133 Free PMC article. - The dilemma of neuroprotection trials in times of successful endovascular recanalization.
Schmidt-Pogoda A, Kaesmacher J, Bonberg N, Werring N, Strecker JK, Koecke MHM, Beuker C, Gralla J, Meier R, Wiendl H, Minnerup H, Fischer U, Minnerup J. Schmidt-Pogoda A, et al. Front Neurol. 2024 Apr 9;15:1383494. doi: 10.3389/fneur.2024.1383494. eCollection 2024. Front Neurol. 2024. PMID: 38654740 Free PMC article. - Uncontrolled pain: a call for better study design.
Hyndman TH, Bowden RS, Woodward AP, Pang DSJ, Hampton JO. Hyndman TH, et al. Front Vet Sci. 2024 Feb 14;11:1328098. doi: 10.3389/fvets.2024.1328098. eCollection 2024. Front Vet Sci. 2024. PMID: 38420206 Free PMC article. Review.
References
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 2005. June;25(6):713–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources