2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer - PubMed (original) (raw)
Review
doi: 10.1089/thy.2015.0020.
Erik K Alexander 2, Keith C Bible 3, Gerard M Doherty 4, Susan J Mandel 5, Yuri E Nikiforov 6, Furio Pacini 7, Gregory W Randolph 8, Anna M Sawka 9, Martin Schlumberger 10, Kathryn G Schuff 11, Steven I Sherman 12, Julie Ann Sosa 13, David L Steward 14, R Michael Tuttle 15, Leonard Wartofsky 16
Affiliations
- PMID: 26462967
- PMCID: PMC4739132
- DOI: 10.1089/thy.2015.0020
Review
2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer
Bryan R Haugen et al. Thyroid. 2016 Jan.
Abstract
Background: Thyroid nodules are a common clinical problem, and differentiated thyroid cancer is becoming increasingly prevalent. Since the American Thyroid Association's (ATA's) guidelines for the management of these disorders were revised in 2009, significant scientific advances have occurred in the field. The aim of these guidelines is to inform clinicians, patients, researchers, and health policy makers on published evidence relating to the diagnosis and management of thyroid nodules and differentiated thyroid cancer.
Methods: The specific clinical questions addressed in these guidelines were based on prior versions of the guidelines, stakeholder input, and input of task force members. Task force panel members were educated on knowledge synthesis methods, including electronic database searching, review and selection of relevant citations, and critical appraisal of selected studies. Published English language articles on adults were eligible for inclusion. The American College of Physicians Guideline Grading System was used for critical appraisal of evidence and grading strength of recommendations for therapeutic interventions. We developed a similarly formatted system to appraise the quality of such studies and resultant recommendations. The guideline panel had complete editorial independence from the ATA. Competing interests of guideline task force members were regularly updated, managed, and communicated to the ATA and task force members.
Results: The revised guidelines for the management of thyroid nodules include recommendations regarding initial evaluation, clinical and ultrasound criteria for fine-needle aspiration biopsy, interpretation of fine-needle aspiration biopsy results, use of molecular markers, and management of benign thyroid nodules. Recommendations regarding the initial management of thyroid cancer include those relating to screening for thyroid cancer, staging and risk assessment, surgical management, radioiodine remnant ablation and therapy, and thyrotropin suppression therapy using levothyroxine. Recommendations related to long-term management of differentiated thyroid cancer include those related to surveillance for recurrent disease using imaging and serum thyroglobulin, thyroid hormone therapy, management of recurrent and metastatic disease, consideration for clinical trials and targeted therapy, as well as directions for future research.
Conclusions: We have developed evidence-based recommendations to inform clinical decision-making in the management of thyroid nodules and differentiated thyroid cancer. They represent, in our opinion, contemporary optimal care for patients with these disorders.
Figures
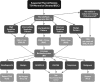
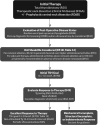
**FIG. 1.
Algorithm for evaluation and management of patients with thyroid nodules based on US pattern and FNA cytology. R, recommendation in text.
**FIG. 2.
ATA nodule sonographic patterns and risk of malignancy.
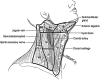
**FIG. 3.
Lymph node compartments separated into levels and sublevels. Level VI contains the thyroid gland, and the adjacent nodes bordered superiorly by the hyoid bone, inferiorly by the innominate (brachiocephalic) artery, and laterally on each side by the carotid sheaths. The level II, III, and IV nodes are arrayed along the jugular veins on each side, bordered anteromedially by level VI and laterally by the posterior border of the sternocleidomastoid muscle. The level III nodes are bounded superiorly by the level of the hyoid bone and inferiorly by the cricoid cartilage; levels II and IV are above and below level III, respectively. The level I node compartment includes the submental and submandibular nodes, above the hyoid bone, and anterior to the posterior edge of the submandibular gland. Finally, the level V nodes are in the posterior triangle, lateral to the lateral edge of the sternocleidomastoid muscle. Levels I, II, and V can be further subdivided as noted in the figure. The inferior extent of level VI is defined as the suprasternal notch. Many authors also include the pretracheal and paratracheal superior mediastinal lymph nodes above the level of the innominate artery (sometimes referred to as level VII) in central neck dissection (341).
**FIG. 4.
Risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy. The risk of structural disease recurrence associated with selected clinico-pathological features are shown as a continuum of risk with percentages (ranges, approximate values) presented to reflect our best estimates based on the published literature reviewed in the text. In the left hand column, the three-tiered risk system proposed as the Modified Initial Risk Stratification System is also presented to demonstrate how the continuum of risk estimates informed our modifications of the 2009 ATA Initial Risk System (see Recommendation 48). *While analysis of BRAF and/or TERT status is not routinely recommended for initial risk stratification, we have included these findings to assist clinicians in proper risk stratification in cases where this information is available. FTC, follicular thyroid cancer; FV, follicular variant; LN, lymph node; PTMC, papillary thyroid microcarcinoma; PTC, papillary thyroid cancer.
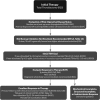
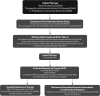
**FIG. 5.
Clinical decision-making and management recommendations in ATA low-risk DTC patients that have undergone total thyroidectomy. R, recommendation in text.
**FIG. 6.
Clinical decision-making and management recommendations in ATA low risk DTC patients that have undergone less than total thyroidectomy (lobectomy or lobectomy with isthmusectomy). R, recommendation in text.
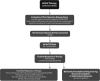
**FIG. 7.
Clinical decision-making and management recommendations in ATA intermediate risk DTC patients that have undergone total thyroidectomy. R, recommendation in text.
**FIG. 8.
Clinical decision-making and management recommendations in ATA high risk DTC patients that have undergone total thyroidectomy and have no gross residual disease remaining in the neck. R, recommendation in text.
Comment in
- A "new/old method" for TSH stimulation: could a third way to prepare DTC patients for (131)I remnant ablation possibly exist?
Giovanella L, Piccardo A. Giovanella L, et al. Eur J Nucl Med Mol Imaging. 2016 Feb;43(2):221-223. doi: 10.1007/s00259-015-3245-9. Epub 2015 Nov 17. Eur J Nucl Med Mol Imaging. 2016. PMID: 26572763 No abstract available. - 2015 American Thyroid Association Guidelines for Thyroid Nodules and Differentiated Thyroid Cancer and Their Implementation in Various Care Settings.
Pitoia F, Miyauchi A. Pitoia F, et al. Thyroid. 2016 Feb;26(2):319-21. doi: 10.1089/thy.2015.0530. Epub 2015 Dec 17. Thyroid. 2016. PMID: 26576627 No abstract available. - Risk of malignancy in 1502 solid thyroid nodules >1 cm using the new ultrasonographic classification of the American Thyroid Association.
Rosario PW, da Silva AL, Nunes MS, Ribeiro Borges MA, Mourão GF, Calsolari MR. Rosario PW, et al. Endocrine. 2017 May;56(2):442-445. doi: 10.1007/s12020-016-1163-7. Epub 2016 Nov 12. Endocrine. 2017. PMID: 27837438 No abstract available. - The "broken chair" in patients with differentiated thyroid cancer.
La Greca A, Pitoia F, Tuttle RM. La Greca A, et al. Endocrine. 2017 Aug;57(2):359-360. doi: 10.1007/s12020-017-1345-y. Epub 2017 Jun 14. Endocrine. 2017. PMID: 28616850 No abstract available.
Similar articles
- 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum.
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. Alexander EK, et al. Thyroid. 2017 Mar;27(3):315-389. doi: 10.1089/thy.2016.0457. Thyroid. 2017. PMID: 28056690 - Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer.
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, et al. Thyroid. 2009 Nov;19(11):1167-214. doi: 10.1089/thy.2009.0110. Thyroid. 2009. PMID: 19860577 - Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer.
Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force. Francis GL, et al. Thyroid. 2015 Jul;25(7):716-59. doi: 10.1089/thy.2014.0460. Thyroid. 2015. PMID: 25900731 Free PMC article. Review. - 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer.
Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. Bible KC, et al. Thyroid. 2021 Mar;31(3):337-386. doi: 10.1089/thy.2020.0944. Thyroid. 2021. PMID: 33728999 Free PMC article. - Implementing Key Changes in the American Thyroid Association 2015 Thyroid Nodules/Differentiated Thyroid Cancer Guidelines Across Practice Types.
Twining CL, Lupo MA, Tuttle RM. Twining CL, et al. Endocr Pract. 2018 Sep;24(9):833-840. doi: 10.4158/EP-2018-0130. Endocr Pract. 2018. PMID: 30308136 Review.
Cited by
- Treatment outcomes in patients with papillary thyroid cancer undergoing radiofrequency ablation of metastatic lymph nodes.
LaForteza A, Persons E, Hussein M, Luo X, Issa PP, Jishu J, Shama M, Toraih E, Kandil E. LaForteza A, et al. Gland Surg. 2024 Oct 31;13(10):1752-1758. doi: 10.21037/gs-24-285. Epub 2024 Oct 26. Gland Surg. 2024. PMID: 39544984 Free PMC article. - Laboratory parameters-based logistic regression models for rapid screening of thyroid nodules.
Liu M, Zhao J, Zhang J, Zhang R. Liu M, et al. Gland Surg. 2024 Oct 31;13(10):1673-1683. doi: 10.21037/gs-24-227. Epub 2024 Oct 26. Gland Surg. 2024. PMID: 39544979 Free PMC article. - The differences between unilateral multifocality and bilateral multifocality in papillary thyroid carcinoma: a retrospective cohort study.
Wu Z, Gong H, Jiang Y, Jiang T, Su A. Wu Z, et al. Gland Surg. 2024 Oct 31;13(10):1684-1692. doi: 10.21037/gs-24-147. Epub 2024 Oct 26. Gland Surg. 2024. PMID: 39544976 Free PMC article. - ASO Author Reflections: Addressing Radioactive Iodine Recommendations in Low-to-Intermediate Risk Papillary Thyroid Cancer.
Palacardo F, Finnerty B. Palacardo F, et al. Ann Surg Oncol. 2024 Nov 14. doi: 10.1245/s10434-024-16514-z. Online ahead of print. Ann Surg Oncol. 2024. PMID: 39542967 No abstract available. - Deep learning-based automatic pipeline system for predicting lateral cervical lymph node metastasis in patients with papillary thyroid carcinoma using computed tomography: A multi-center study.
Yu P, Wang C, Zhang H, Zheng G, Jia C, Liu Z, Wang Q, Mu Y, Yang X, Mao N, Song X. Yu P, et al. Chin J Cancer Res. 2024 Oct 30;36(5):545-561. doi: 10.21147/j.issn.1000-9604.2024.05.07. Chin J Cancer Res. 2024. PMID: 39539818 Free PMC article.
References
- Vander JB, Gaston EA, Dawber TR. 1968. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med 69:537–540 - PubMed
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. 1977. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 7:481–493 - PubMed
- Tan GH, Gharib H. 1997. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 126:226–231 - PubMed
- Guth S, Theune U, Aberle J, Galach A, Bamberger CM. 2009. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 39:699–706 - PubMed
- Hegedus L. 2004. Clinical practice. The thyroid nodule. N Engl J Med 351:1764–1771 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials