LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/β-catenin signalling in neuroblastoma - PubMed (original) (raw)
. 2015 Nov 24;6(37):40053-67.
doi: 10.18632/oncotarget.5548.
S Chockalingam 1, Zsombor Melegh 2, Alexander Greenhough 3, Sally Malik 1, Marianna Szemes 1, Ji Hyun Park 1, Abderrahmane Kaidi 1, Li Zhou 4, Daniel Catchpoole 4, Rhys Morgan 3, David O Bates 5, Peter David Gabb 1, Karim Malik 1
Affiliations
- PMID: 26517508
- PMCID: PMC4741879
- DOI: 10.18632/oncotarget.5548
LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/β-catenin signalling in neuroblastoma
Gabriella Cunha Vieira et al. Oncotarget. 2015.
Erratum in
- Correction: LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/β-catenin signalling in neuroblastoma.
Vieira GC, Chockalingam S, Melegh Z, Greenhough A, Malik S, Szemes M, Park JH, Kaidi A, Zhou L, Catchpoole D, Morgan R, Bates DO, Gabb PJ, Malik K. Vieira GC, et al. Oncotarget. 2017 May 9;8(19):32381. doi: 10.18632/oncotarget.17685. Oncotarget. 2017. PMID: 28499332 Free PMC article. No abstract available.
Abstract
LGR5 is a marker of normal and cancer stem cells in various tissues where it functions as a receptor for R-spondins and increases canonical Wnt signalling amplitude. Here we report that LGR5 is also highly expressed in a subset of high grade neuroblastomas. Neuroblastoma is a clinically heterogenous paediatric cancer comprising a high proportion of poor prognosis cases (~40%) which are frequently lethal. Unlike many cancers, Wnt pathway mutations are not apparent in neuroblastoma, although previous microarray analyses have implicated deregulated Wnt signalling in high-risk neuroblastoma. We demonstrate that LGR5 facilitates high Wnt signalling in neuroblastoma cell lines treated with Wnt3a and R-spondins, with SK-N-BE(2)-C, SK-N-NAS and SH-SY5Y cell-lines all displaying strong Wnt induction. These lines represent MYCN-amplified, NRAS and ALK mutant neuroblastoma subtypes respectively. Wnt3a/R-Spondin treatment also promoted nuclear translocation of β-catenin, increased proliferation and activation of Wnt target genes. Strikingly, short-interfering RNA mediated knockdown of LGR5 induces dramatic Wnt-independent apoptosis in all three cell-lines, accompanied by greatly diminished phosphorylation of mitogen/extracellular signal-regulated kinases (MEK1/2) and extracellular signal-regulated kinases (ERK1/2), and an increase of BimEL, an apoptosis facilitator downstream of ERK. Akt signalling is also decreased by a Rictor dependent, PDK1-independent mechanism. LGR5 expression is cell cycle regulated and LGR5 depletion triggers G1 cell-cycle arrest, increased p27 and decreased phosphorylated retinoblastoma protein. Our study therefore characterises new cancer-associated pathways regulated by LGR5, and suggest that targeting of LGR5 may be of therapeutic benefit for neuroblastomas with diverse etiologies, as well as other cancers expressing high LGR5.
Keywords: LGR5; MEK/ERK; Wnt/β-catenin; cell survival; neuroblastoma.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures
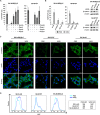
Figure 1. Expression of LGR5 in neuroblastoma
A. Kaplan-Meier survival curves derived from 2 independent microarray analyses of neuroblastomas showing the association of high LGR5 mRNA expression with poor prognosis. Datasets used are from reference [27] (left) and [26] (right), obtained using the R2 Genomics Analysis and Visualization Platform (
). B. Immunoblotting showing elevated expression of LGR5 in primary NBs. Fetal Adrenal protein is shown as a normal control(FA). C. Immunohistochemistry demonstrating prominent cytoplasmic staining in poorly differentiated NBs (top) and little or no staining in differentiating NBs (bottom). A section of normal colon where interspersed LGR5 positive cells are located at the bottom of crypts is shown as a control. D. Immunoblotting showing elevated expression of LGR5 in cell-lines. The colorectal cancer cell line LOVO is shown for comparison.
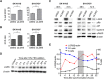
Figure 2. Wnt3a and R-spondin responsiveness of neuroblastoma cell lines
A. TOPFLASH reporter assay for SK-N-BE(2)-C and SH-SY5Y showing increased luciferase activity upon co-stimulation with Wnt3a and Rspos 1–3 ( p < 5.0e-04 and p < 5.0e-03 respectively). B. TOPFLASH reporter assay combined with control siRNA, LGR5 or β-catenin targeting siRNAs, demonstrating requirement for LGR5 for reporter activity ( p < 0.05). Reporter assays were conducted at least twice in triplicate. PBS: Phosphate-buffered saline, WR2: Wnt3a/Rspo2 treatment. C. Confocal microscopy of β-catenin nuclear translocation in SK-N-BE(2)-C, SH-SY5Y and SK-N-AS cells on addition of Wnt3a/Rspo2. Green fluorescent staining shows β-catenin and cells were counterstained with DAPI (blue) for nuclei control D. Flow cytometry analysis of NB lines treated with Wnt3a/Rspo2 and labelled with Ki67 to measure proliferation. Note the shift and increased median fluorescence intensity (MFI) accompanying treatment. Note that x-axes are in log scale.
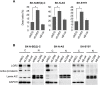
Figure 3. LGR5 knockdown-induced cell death is independent of Wnt/β-catenin signalling pathway
A. SK-N-BE(2)-C, SK-N-AS and SH-SY5Y cells were transfected with siRNAs targeting LGR5 or β-catenin. LGR5 knockdown led to dramatic cell death in all three cell lines tested ( p < 0.05), but β-catenin depletion did not. B. Immunoblots confirming knockdowns and the absence of dephosphorylated (active) β-catenin. C: cytoplasmic, N: nuclear.
Figure 4. Depletion of LGR5 induces apoptosis in neuroblastoma cells
Knockdown of LGR5 with independent siRNAs induces apoptosis shown by cell-counts, increased cleaved PARP (cPARP), and rescue by the caspase 3 inhibitor QVD A. SK-N-AS cells and B. SH-SY5Y cells. Asterisks denote p < 0.05, and assays are representative of at least 3 biological replicates. Membrane asymmetry assessment of apoptosis in C. SK-N-AS cells and D. SH-SY5Y cells. EA, early apoptosis; LA, late apoptosis.
Figure 5. LGR5 regulates MEK/ERK and Akt signalling
Immunoblotting demonstrating A. decreases in p-ERK1/2 (T202/Y204) and p-MEK1/2 (S217/221), B. Elevated Bim-EL and C. altered Akt phosphorylation and Rictor levels accompanying LGR5 knockdown.
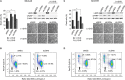
Figure 6. LGR5 is required for G1 to S phase transition
A. Increased sub-G0/G1 cells following LGR5 knockdown of SK-N-AS and SH-SY5Y cells demonstrated by flow cytometry. Asterisks denote p < 0.05. B. Cell cycle analysis of LGR5 knockdowns. After 48 hrs of transfection, G1 arrest of cells is apparent through comparison of G1-S phase ratios. Asterisks denote p < 0.05. C. Immunoblotting analysis of cell cycle regulatory proteins confirming G1 arrest accompanying LGR5 knockdown.D. Immunoblot demonstrating cell cycle dependent changes in LGR5 expression in SH-SY5Y cells. E. Quantification of LGR5 protein levels (relative to β-actin) plotted against percentage of cells in G1- or S-phase.
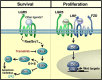
Figure 7. A model showing regulatory modalities for LGR5 in neuroblastoma
Pro-survival signalling of MEK/ERK activities which are R-Spondin independent are shown on the left, and may be inhibited by MEK/ERK inhibitors such as Trametinib. Proliferative Wnt signalling in the presence of Wnt/Rspos is shown on the right. FZD: Frizzled receptors; LRP: Low Density Lipoprotein Receptor-Related Proteins, β-cat: β-catenin, TCF: T-cell factor/lymphoid enhancer factor.
Similar articles
- Wnt Signalling Drives Context-Dependent Differentiation or Proliferation in Neuroblastoma.
Szemes M, Greenhough A, Melegh Z, Malik S, Yuksel A, Catchpoole D, Gallacher K, Kollareddy M, Park JH, Malik K. Szemes M, et al. Neoplasia. 2018 Apr;20(4):335-350. doi: 10.1016/j.neo.2018.01.009. Epub 2018 Mar 3. Neoplasia. 2018. PMID: 29505958 Free PMC article. - TRIM59 knockdown inhibits cell proliferation by down-regulating the Wnt/β-catenin signaling pathway in neuroblastoma.
Chen G, Chen W, Ye M, Tan W, Jia B. Chen G, et al. Biosci Rep. 2019 Jan 18;39(1):BSR20181277. doi: 10.1042/BSR20181277. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30389710 Free PMC article. - Wnt signalling is a bi-directional vulnerability of cancer cells.
Duffy DJ, Krstic A, Schwarzl T, Halasz M, Iljin K, Fey D, Haley B, Whilde J, Haapa-Paananen S, Fey V, Fischer M, Westermann F, Henrich KO, Bannert S, Higgins DG, Kolch W. Duffy DJ, et al. Oncotarget. 2016 Sep 13;7(37):60310-60331. doi: 10.18632/oncotarget.11203. Oncotarget. 2016. PMID: 27531891 Free PMC article. - The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength.
de Lau W, Peng WC, Gros P, Clevers H. de Lau W, et al. Genes Dev. 2014 Feb 15;28(4):305-16. doi: 10.1101/gad.235473.113. Genes Dev. 2014. PMID: 24532711 Free PMC article. Review. - Lgr5 in cancer biology: functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy.
Xu L, Lin W, Wen L, Li G. Xu L, et al. Stem Cell Res Ther. 2019 Jul 29;10(1):219. doi: 10.1186/s13287-019-1288-8. Stem Cell Res Ther. 2019. PMID: 31358061 Free PMC article. Review.
Cited by
- Nicotinamide Riboside Promotes the Proliferation of Endogenous Neural Stem Cells to Repair Spinal Cord Injury.
Zhang J, Shang J, Ding H, Li W, Li Z, Yuan Z, Zheng H, Lou Y, Wei Z, Zhou H, Feng S, Kong X, Ran N. Zhang J, et al. Stem Cell Rev Rep. 2024 Oct;20(7):1854-1868. doi: 10.1007/s12015-024-10747-x. Epub 2024 Jun 28. Stem Cell Rev Rep. 2024. PMID: 38941038 - Lgr4 is crucial for skin carcinogenesis by regulating MEK/ERK and Wnt/β-catenin signaling pathways.
Xu P, Dang Y, Wang L, Liu X, Ren X, Gu J, Liu M, Dai X, Ye X. Xu P, et al. Cancer Lett. 2016 Dec 28;383(2):161-170. doi: 10.1016/j.canlet.2016.09.005. Epub 2016 Sep 30. Cancer Lett. 2016. PMID: 27693558 Free PMC article. - ERK is a Pivotal Player of Chemo-Immune-Resistance in Cancer.
Salaroglio IC, Mungo E, Gazzano E, Kopecka J, Riganti C. Salaroglio IC, et al. Int J Mol Sci. 2019 May 21;20(10):2505. doi: 10.3390/ijms20102505. Int J Mol Sci. 2019. PMID: 31117237 Free PMC article. Review. - LGR5 enhances the osteoblastic differentiation of MC3T3-E1 cells through the Wnt/β-catenin pathway.
Yu W, Xie CR, Chen FC, Cheng P, Yang L, Pan XY. Yu W, et al. Exp Ther Med. 2021 Aug;22(2):889. doi: 10.3892/etm.2021.10321. Epub 2021 Jun 17. Exp Ther Med. 2021. PMID: 34194567 Free PMC article. - Overexpression of MicroRNA-216a Suppresses Proliferation, Migration, and Invasion of Glioma Cells by Targeting Leucine-Rich Repeat-Containing G Protein-Coupled Receptor 5.
Zhang J, Xu K, Shi L, Zhang L, Zhao Z, Xu H, Liang F, Li H, Zhao Y, Xu X, Tian Y. Zhang J, et al. Oncol Res. 2017 Sep 21;25(8):1317-1327. doi: 10.3727/096504017X14874323871217. Epub 2017 Mar 2. Oncol Res. 2017. PMID: 28256193 Free PMC article.
References
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nature Reviews Cancer. 2003;3:203–216. - PubMed
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
- Chen YY, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang LL, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–U956. - PubMed
- Shukla N, Ameur N, Yilmaz I, Nafa K, Lau CY, Marchetti A, Borsu L, Barr FG, Ladanyi M. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:748–757. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous