Lysyl oxidase propeptide stimulates osteoblast and osteoclast differentiation and enhances PC3 and DU145 prostate cancer cell effects on bone in vivo - PubMed (original) (raw)
Lysyl oxidase propeptide stimulates osteoblast and osteoclast differentiation and enhances PC3 and DU145 prostate cancer cell effects on bone in vivo
Mona Alsulaiman et al. J Cell Commun Signal. 2016 Mar.
Abstract
Lysyl oxidase pro-enzyme is secreted by tumor cells and normal cells as a 50 kDa pro-enzyme into the extracellular environment where it is cleaved into the ~30 kDa mature enzyme (LOX) and 18 kDa pro-peptide (LOX-PP). Extracellular LOX enzyme activity is required for normal collagen and elastin extracellular cross-linking and maturation of the extracellular matrix. Extracellular LOX-PP acts as a tumor suppressor and can re-enter cells from the extracellular environment to induce its effects. The underlying hypothesis is that LOX-PP has the potential to promote bone cell differentiation, while inhibiting cancer cell effects in bone. Here we investigate the effect of LOX-PP on bone marrow cell proliferation and differentiation towards osteoblasts or osteoclasts, and LOX-PP modulation of prostate cancer cell conditioned media-induced alterations of proliferation and differentiation of bone marrow cells in vitro. Effects of overexpression of rLOX-PP in DU145 and PC3 prostate cancer cell lines on bone structure in vivo after intramedullary injections were determined. Data show that prostate cancer cell conditioned media inhibited osteoblast differentiation in bone marrow-derived cells, which was reversed by rLOX-PP treatment. Prostate cancer conditioned media stimulated osteoclast differentiation which was further enhanced by rLOX-PP treatment. rLOX-PP stimulated osteoclast differentiation by inhibiting OPG expression, up-regulating CCN2 expression, and increasing osteoclast fusion. In vivo studies indicate that rLOX-PP expression by PC3 cells implanted into the tibia of mice further enhanced PC3 cell ability to resorb bone, while rLOX-PP expression in DU145 cells resulted in non-significant increases in net bone formation. rLOX-PP enhances both osteoclast and osteoblast differentiation. rLOX-PP may serve to enhance coupling interactions between osteoclasts and osteoblasts helping to maintain a normal bone turnover in health, while contributing to bone abnormalities in disease.
Keywords: Bone; Differentiation; Lysyl oxidase; Lysyl oxidase propeptide; Osteoblast; Osteoclast.
Figures
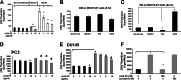
Fig. 1
rLOX-PP effects on prostate cancer cell conditioned media induced proliferation of a-c MC3T3-E1 osteoblasts, and BMSCs d-f, and effects of rLOX-PP are specific f: MC3T3 cultures were serum-depleted for 24 h and then a treated with DU145 conditioned medium for 8 h in the presence of rLOX-PP (0–10 μg/ml equivalent to 0–555 nM) (white bars); or non-conditioned serum-free α-MEM medium (black bars) or with MC3T3 cell conditioned serum-free α-MEM medium (hatched bars). Data are cpm/well ± S.E.M. b and c. MC3T3 cells were treated with conditioned media from PC3 cells, or with positive control FGF-2 (1 ng/ml) for 6 or 24 h, and [3H] -thymidine incorporation determined; (*, p < 0.05). BMSCs were placed in differentiation medium for 4 days. On Day 8 the cells were serum-depleted for 24 h and then treated with d PC3 or e DU145 conditioned medium (hatched bars) or non-conditioned medium plus rLOX-PP (0–10 μg/ml) (black bars), and subjected to [3H]thymidine incorporation assays. Data are cpm/well ±S.E.M combined results from three experiments (n = 3, *, p˂0.05; #, p < 0.05 compared to no rLOX-PP control). f MC3T3 cultures were serum-starved for 24 h and then induced to proliferate with DU145 conditioned medium (hatched bars) in the presence or absence of either rLOX-PP (10 μg/ml equivalent to 555 nM) or a molar equivalent of lysozyme (8 μg/ml). Non-conditioned serum-free α-MEM medium was used as a control group in this experiment (black bar). [3H]thymidine incorporation was performed as indicated in Materials and Methods. Data are cpm/well ±S.D., performed once in triplicate (n = 3, *, p˂0.05; #, p < 0.05 compared to no rLOX-PP control; one-way ANOVA).
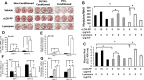
Fig. 2
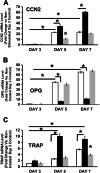
Alizarin red staining of bone marrow stromal cells treated with prostate cancer conditioned medium in the presence or absence of rLOX-PP (a – c) and expression of markers (d-g) : BMSCs were plated and grown under osteoblast induction conditions as described in Fig. 1 and Materials and Methods. Cells were then treated with 50 % non-conditioned medium or 50 % DU145- or PC3 conditioned medium in the presence or absence of rLOX-PP (10 μg/ml) or lysozyme (8 μg/ml of medium) in 5 % serum and 10 nM dexamethasone, 50 μg/ml ascorbic acid, and 8 mM β-glycerol phosphate. The BMSCs were refed every 48 h with the same respective media for 28 days. Alizarin Red stained cultures (a), and intensity of staining was measured using a Versadoc 3000 imaging system (b); absorbance determined after elution of Alizarin Red dye at 405 nm (c); black bars, non-conditioned media; hatched bars, DU145 conditioned media; white bars, PC3 conditioned media. Data are mean ± S.D. with n = 3, *, p˂0.05. (d-g) Cells were grown for 7 or 14 days in respective differentiation medium, and relative RNA expressions determined from cells treated with non-conditioned medium (control, black bars), or 50 % DU145 or 50 % PC3 conditioned media in the absence (hatched bars) or presence (white bars) of rLOX-PP (10 μg/ml of medium equivalent to 555 nM). Fold changes in mRNA levels relative to the day 7 non-conditioned medium control all normalized to β-actin mRNA levels are shown (d-g). Data are means ±S.D., n = 3, *,p˂0.05 compared to the respective non-conditioned media control.
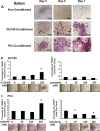
Fig. 3
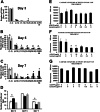
Time course a and effects of DU145 b and PC3 c conditioned medium treatment on osteoclast development in BMSCs cultures: a BMSCs were plated and induced to undergo osteoblast differentiation as described in Materials and Methods. On day 8 cells were serum starved for 24 h. The control group was treated with 50 % non-conditioned serum free α-MEM medium or 50 % DU145 or PC3 cell conditioned media, with the remaining 50 % consisting of α-MEM containing 10 % FBS and osteogenic factors. Respective media were replaced every 48 h. Cultures were fixed in 10 % formaldehyde at room temperature for 30 min and stained for TRAP as described in Materials and Methods. (b and c) BMSCs were treated with DU145- b or PC3- (c) conditioned medium and increasing concentrations of either rLOX-PP or lysozyme (negative control). Cultures were fixed and TRAP stained (Materials and Methods) and digital images from each triplicate well were captured from four randomly selected areas with a Zeiss Axiovert 200 microscope 100X magnification. Data are expressed as the percentage of the well surface area that is covered by osteoclasts, and are compared to the respective non-treated controls (means +/− SD, n = 3, *, p < 0.05 compared to respective zero control). Statistical analysis was carried out using one way ANOVA. Representative images of the TRAP stained cells are shown below each data point (scale bars =220 μm).
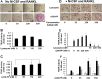
Fig. 4
Effect of rLOX-PP treatment on osteoclast development in the absence (a-c) or presence (d-f) of M-CSF and RANKL**:** BMSCs were plated in 6 well plates. At 80 % visual confluence they were treated with non-conditioned medium containing 5 % serum and osteogenic factors as described in Materials and Methods with increasing concentrations of rLOX-PP or lysozyme, or vehicle control (zero rLOX-PP or lysozyme). Cultures were fixed in 10 % formaldehyde and subjected to TRAP staining on Day 5. Digital images were collected and analyzed as in Fig. 3, scale bar, 200 μm a. Data in (b and c) are TRAP enzyme activity in the culture supernatant from the same cultures and absorbance measured at 540 nm +/− SD, *, p < 0.05, n = 3. d BMSCs were plated in 6-well plates in at 5 × 106 cells per well and grown in α-MEM supplemented with 10 % FBS. Osteoclast differentiation was induced with murine colony-stimulating factor M-CSF, 50 ng/mL and RANKL in the presence of increasing concentrations of either rLOX-PP or equivalent concentrations of lysozyme. BMSCs were fixed and TRAP-stained and representative images images are shown in d, scale bar, 200 μm. e TRAP enzyme activity in the culture supernatant from the same cultures was measured. Bars at the extreme left of Fig. 4e is TRAP activity from Day 2 culture supernatants in cells treated with M-CSF/RANKL but without rLOX-PP and indicates little or no TRAP activity at this time point as expected. In f the experiment was repeated as a function of both rLOX-PP concentration and time of culture. White bars, Day 3; hatched bars, Day 4; black bars, Day 5, gray bars, Day 7. Data are means ±S.D.; *, p˂0.05 compared to no addition of rLOX-PP or lysozyme; n = 3 for each respective group or time point. Statistical analysis was carried out using one way ANOVA.
Fig. 5
Regulation of mRNA expression of osteoclast differentiation markers by rLOX-PP**_._** BMSCs were plated in 6-well plates in a 5 × 106 cells per well and grown in α-MEM supplemented with 10 % fetal bovine serum (FBS). Osteoclast differentiation was induced with murine colony-stimulating factor (M-CSF, 50 ng/mL) and RANKL (30 ng/mL) in the presence or absence of rLOX-PP 550 nM (10 μg) or an equivalent molar concentration of lysozyme (8 μg/ml) as described in Materials and Methods. RNA was isolated at intervals and assessed by qPCR for the target markers and β-actin as the normalization control. The fold changes in expression of markers were relative to the Day 3 no treatment control group which consisted of only M-CSF/RANKL additions. Data represent means ± S.D., n = 3; *p˂0.05; white bars, M-CSF/RANKL only; black bars, plus rLOX-PP; gray bars, plus lysozyme. Statistical analysis was carried out as described in Materials and Methods
Fig. 6
Effect of rLOX-PP treatment of BMSCs on osteoclast number and fusion (a-d), and assessment of apoptosis (e-g): Bone marrow cells were induced with M-CSF (50 ng/mL) RANKL, (30 ng/mL) in the presence of either 550 nM (10 μg) rLOX- or 550 nM (8 μg) lysozyme. Culture media were changed every other day and rM-CSF and rRANKL were added to the growth medium throughout the experiment. The cells were grown for either 3, 5 or 7 days after initiation of rLOX-PP treatment. This was followed by TRAP staining of cell layers. Digital images of 20–36 randomly selected non-overlapping areas of each well were taken for analyses of TRAP staining with inverted microscope at 100X magnification. The total number of osteoclasts per well in each treatment group was calculated. The numbers of nuclei in each osteoclast were counted and the data were divided into 6 groups. The percentage of cells falling into each category was calculated and compared among different treatment conditions (a-d). White bars, cells treated with M-CSF and RANKL only; black bars, cells treated with M-CSF, RANKL and rLOX-PP; gray bars, cells treated with M-CSF, RANKL, and lysozyme. Data represent mean ± S.D., n = 3, *p˂0.05. (e-g) Bone marrow cells were grown under conditions to promote osteoclast development in the presence or absence of rLOX-PP or lysozyme as in (A-D). Caspase-3 activity was measured in cell lysates. Data are means ±S.D., n = 3, *p˂0.05 compared to no treatment controls
Fig. 7
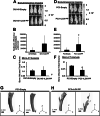
Intra-tibial injection of DU145 and PC3 ectopically overexpressing rLOX-PP promotes growth and colonization leading to bone remodeling: Representative mice (n = 6/condition) showing bioluminescence imaging after 6 week intra-tibial injection of DU145-Empty or rLOX-PP cells a, and luciferase-bioluminescence +/− SD b. Luciferase expressing DU145 cells were infected by transduction of EF1α-LOX-PP-myc-his-UBC-GFP (designated as rLOX-PP) and empty vector EF1α-Empty-UBC-GFP (designated as Empty) lentivirus particles. These cells (2.5 X 105) were injected into the tibia of mice (n = 6) showing increase in tumor growth and colonization in the cells expressing rLOX-PP compared to Empty. c Micro-CT analysis of tibia shows no significant effect on bone volume/total volume in rLOX-PP expressing DU145 cells compared to empty controls. d Representative mice (n = 9/condition) showing bioluminescence imaging by IVIS after 8 weeks intra-tibial injection of PC3-Empty or rLOX-PP expressing cells, and e quantitation of bioluminescence by IVIS +/−SD, *, p < 0.05 compared to Empty expressing cells. f Micro-CT analysis of tibia shows decrease in the ratio of bone volume/total volume in rLOX-PP expressing group compared to empty; +/− SD; n = 9, * p < 0.05, analysis by one way ANOVA. Representative 3-D reconstruction images of tibia injected with PC3-Empty cells and g, and of tibia injected with PC3-rLOX-PP cells h at sacrifice on day 42.
Similar articles
- Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways.
Bais MV, Ozdener GB, Sonenshein GE, Trackman PC. Bais MV, et al. Oncogene. 2015 Apr 9;34(15):1928-37. doi: 10.1038/onc.2014.147. Epub 2014 Jun 2. Oncogene. 2015. PMID: 24882580 Free PMC article. - Determination of cell uptake pathways for tumor inhibitor lysyl oxidase propeptide.
Ozdener GB, Bais MV, Trackman PC. Ozdener GB, et al. Mol Oncol. 2016 Jan;10(1):1-23. doi: 10.1016/j.molonc.2015.07.005. Epub 2015 Aug 6. Mol Oncol. 2016. PMID: 26297052 Free PMC article. - Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts.
Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Vora SR, et al. J Biol Chem. 2010 Mar 5;285(10):7384-93. doi: 10.1074/jbc.M109.033597. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048148 Free PMC article. - Functions and Mechanisms of Pro-Lysyl Oxidase Processing in Cancers and Eye Pathologies with a Focus on Diabetic Retinopathy.
Trackman PC, Peymanfar Y, Roy S. Trackman PC, et al. Int J Mol Sci. 2022 May 3;23(9):5088. doi: 10.3390/ijms23095088. Int J Mol Sci. 2022. PMID: 35563478 Free PMC article. Review. - Cellular and molecular effects of growth hormone and estrogen on human bone cells.
Kassem M. Kassem M. APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
Cited by
- Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone.
Trackman PC. Trackman PC. Matrix Biol. 2016 May-Jul;52-54:7-18. doi: 10.1016/j.matbio.2016.01.001. Epub 2016 Jan 6. Matrix Biol. 2016. PMID: 26772152 Free PMC article. Review. - The role of lysyl oxidase, the extracellular matrix and the pre-metastatic niche in bone metastasis.
Gartland A, Erler JT, Cox TR. Gartland A, et al. J Bone Oncol. 2016 Jul 1;5(3):100-103. doi: 10.1016/j.jbo.2016.04.001. eCollection 2016 Sep. J Bone Oncol. 2016. PMID: 27761366 Free PMC article. - Prostate cancer metastasis and soy isoflavones: a dogfight over a bone.
Ajdžanovic V, Filipovic B, Miljic D, Mijatovic S, Maksimovic-Ivanic D, Miler M, Živanovic J, Miloševic V. Ajdžanovic V, et al. EXCLI J. 2019 Feb 19;18:106-126. eCollection 2019. EXCLI J. 2019. PMID: 30956643 Free PMC article. Review. - Lysyl Oxidase and the Tumor Microenvironment.
Wang TH, Hsia SM, Shieh TM. Wang TH, et al. Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review. - Lysyl oxidase family members in urological tumorigenesis and fibrosis.
Li T, Wu C, Gao L, Qin F, Wei Q, Yuan J. Li T, et al. Oncotarget. 2018 Apr 13;9(28):20156-20164. doi: 10.18632/oncotarget.24948. eCollection 2018 Apr 13. Oncotarget. 2018. PMID: 29732010 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous