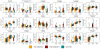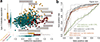Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota - PubMed (original) (raw)
. 2015 Dec 10;528(7581):262-266.
doi: 10.1038/nature15766. Epub 2015 Dec 2.
Falk Hildebrand # 1 2 3, Trine Nielsen # 4, Gwen Falony # 2 5, Emmanuelle Le Chatelier # 6 7, Shinichi Sunagawa 1, Edi Prifti 6 7 8, Sara Vieira-Silva 2 5, Valborg Gudmundsdottir 9, Helle K Pedersen 9, Manimozhiyan Arumugam 4, Karsten Kristiansen 10, Anita Yvonne Voigt 1 11 12, Henrik Vestergaard 4, Rajna Hercog 1, Paul Igor Costea 1, Jens Roat Kultima 1, Junhua Li 13, Torben Jørgensen 14 15 16, Florence Levenez 6 7, Joël Dore 6 7; MetaHIT consortium; H Bjørn Nielsen 9, Søren Brunak 9 17, Jeroen Raes 2 3 5, Torben Hansen 4 18, Jun Wang 10 13 19 20 21, S Dusko Ehrlich 6 7 22, Peer Bork 1, Oluf Pedersen 4
Collaborators, Affiliations
- PMID: 26633628
- PMCID: PMC4681099
- DOI: 10.1038/nature15766
Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota
Kristoffer Forslund et al. Nature. 2015.
Erratum in
- Corrigendum: Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota.
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J; MetaHIT consortium; Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Forslund K, et al. Nature. 2017 May 3;545(7652):116. doi: 10.1038/nature22318. Nature. 2017. PMID: 28470190 No abstract available.
Abstract
In recent years, several associations between common chronic human disorders and altered gut microbiome composition and function have been reported. In most of these reports, treatment regimens were not controlled for and conclusions could thus be confounded by the effects of various drugs on the microbiota, which may obscure microbial causes, protective factors or diagnostically relevant signals. Our study addresses disease and drug signatures in the human gut microbiome of type 2 diabetes mellitus (T2D). Two previous quantitative gut metagenomics studies of T2D patients that were unstratified for treatment yielded divergent conclusions regarding its associated gut microbial dysbiosis. Here we show, using 784 available human gut metagenomes, how antidiabetic medication confounds these results, and analyse in detail the effects of the most widely used antidiabetic drug metformin. We provide support for microbial mediation of the therapeutic effects of metformin through short-chain fatty acid production, as well as for potential microbiota-mediated mechanisms behind known intestinal adverse effects in the form of a relative increase in abundance of Escherichia species. Controlling for metformin treatment, we report a unified signature of gut microbiome shifts in T2D with a depletion of butyrate-producing taxa. These in turn cause functional microbiome shifts, in part alleviated by metformin-induced changes. Overall, the present study emphasizes the need to disentangle gut microbiota signatures of specific human diseases from those of medication.
Figures
Extended Data Figure 1
A. As a positive control for the meta-analysis pipeline, true signal was removed from the data by randomly reshuffling sample labels. Artificial contrast was thereafter introduced between random groups containing as many such reshuffled samples as were in the original sets of T2D metformin+ (nCHN=15, nMHD=58, nSWE=20) and T2D metformin− (nCHN=56, nMHD=17, nSWE=33) samples in each original study subset, using the genus Akkermansia as an example feature. Samples randomly assigned to the sets of fake “metformin treated” and “control” categories had their Akkermansia genus abundances adjusted to match the scale of the metformin effect on Escherichia genus abundance reported here (metformin-treated samples roughly 150% as likely to have nonzero abundance, with a roughly threefold higher abundance where present), while retaining their dataset origin labels. The full meta-analysis pipeline (study set blocked KWT test, post-hoc WRS test) was applied to these samples. Benjamini-Hochberg-corrected P-values (FDR scores/Q-values) from testing for a metformin effect on Akkermansia abundance are here plotted in logarithmic scale on the vertical axis for 100 randomizations of the entire shuffled dataset, either without (left boxplot) or with (right boxplot) the artificial Akkermansia metformin signal added after shuffling the data to remove original signal. Box plot borders show medians and quartiles, with points outside this range shown as vertical whisker lines and point markers, whiskers extend to 1.58 × interquartile range / sqrt (n). Horizontal guide lines are shown for ease of visualization corresponding to different false discovery rate thresholds. For randomly reshuffled data, as expected no significant contrast is detected, while the artificially introduced signal is reliably detected, roughly matching expectations from the definition of the false discovery rate itself. B. To investigate statistical power for the other medications tracked, five random sub-samplings were made of pairs of medicated and non-medicated samples, at each increasing number of included sample pairs, and the overall analysis replicated for each, testing each genus for significantly (KW-test followed by post-hoc WRS test) differential abundance between cases and controls, at different BH FDR significance cutoffs marked in the figure using different colours. Out of the total number of samples for which medication status was known, equal numbers n of medicated and unmedicated samples were chosen randomly in repeated iterations. This number n was varied up to its largest possible value (smallest of either number of medicated or unmedicated samples in overall dataset) and is what is shown on the horizontal axis. The vertical axis shows number of features significant relative to each cutoff, with standard deviation over each set of five randomized samples shown as error bars. C. The graphs show Intestinibacter and Escherichia median and quartile abundances as boxplots, whiskers extend to 1.58 × interquartile range / sqrt (n), with samples extreme relative to the interquartile range shown as point markers and with samples below detection threshold (DT) plotted at y = 0, in 21 additional T2D metformin+ and 9 additional T2D metformin− samples. Differences in abundance between sample categories are significant (WRS test, BH FDR < 0.1). The samples where Intestinibacter was detected all fall among the 9/30 untreated rather than the 21/30 metformin-treated samples, consistent with severe depletion under treatment, whereas Escherichia abundances increase under treatment, likewise consistent with observations from the main dataset.
Extended Data Figure 2
Differences in physiological variables and microbiome characteristics between Chinese (n=368), Danish MetaHIT (n=383) and Swedish (n=145) gut metagenome sample sets. A. Several participant metadata variables are significantly different between cohorts, of which a subselection is shown here as boxplots displaying median and quartiles, with samples outside this range shown as point markers and whiskers, whiskers extend to 1.58 × interquartile range / sqrt (n). B. In a PCoA ordination of Bray-Curtis distances between samples on bacterial family level, clear differences between samples from the different cohorts become apparent. These are largely explained by taxonomic differences as summarized at the phylum level. C. Boxplots for gut microbial taxa show medians and quartiles of log-transformed read counts for mOTUs summarized at the level of bacterial genera, for the three country subsets across sample categories, with samples outside this range shown as point markers and whiskers, whiskers extend to 1.58 × interquartile range / sqrt (n). For all boxplots, tests for significant differences (Kruskal-Wallis test adjusted for study source) were performed with P-values shown at the head of each figure. Star markers note significance of tests done for each country subset separately.
Extended Data Figure 3
Taxonomic microbiome composition comparison between T2D metformin− (n=106), T2D metformin+ (n=93) and ND CTRL (n=554) gut metagenomes with particular focus on possible taxonomic restoration under metformin treatment for certain taxa. Boxplots show medians and quartiles log-transformed read counts for mOTUs summarized at the level of bacterial genera, for the three country subsets across sample categories, with samples outside this range shown as point markers and whiskers, whiskers extend to 1.58 × interquartile range / sqrt (n). Tests for significant differences (Kruskal-Wallis test adjusted for study source) were performed with P-values shown at the head of each figure. Star markers show results of tests for each country subset separately.
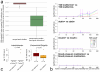
Figure 1. Type 2 diabetes is confounded by metformin treatment
Major treatment effects are seen in multivariate analysis and in classifier performance. A. Projection of genus level gut microbiomes samples from Danish, Chinese and Swedish studies constrained by diabetic state and metformin treatment. Multivariate analysis (dbRDA plot based on Canberra distances between bacterial genera) reveals a T2D dysbiosis which overlaps only in part with taxonomic changes in metformin-treated patients. The ordination projects all T2D metformin+ (n=93, dark red), T2D metformin− (n=106, orange) and ND CTRL (n=554, teal) gut metagenomes, with confounding country effect adjusted for. Bacterial genera which show significant effects of metformin treatment and T2D status compared to ND CTRL, respectively (limited to top five for each), are interpolated into the plane of maximal separation based on their abundances across all samples. Marginal box-/scatterplots show the separation of the constrained projection coordinates (boxes show medians/quartiles, error bars extend to most extreme value within 1.5 interquartile range). The T2D separation is significant (Permanova FDR<0.005) in the joint dataset and independently significant in CHN and MHD samples. The metformin separation is significant (Permanova FDR<0.1; Canberra distances) in MHD and SWE samples. B. Classifying type 2 diabetes and metformin treatment status based on gut microbiome profiles. Support Vector Machine (SVM) classifiers were used to separate T2D metformin+ (n=93), T2D metformin− (n=106) and ND CTRL (n=554) gut metagenomes from each other based on genus-level gut microbiome taxonomic composition. Bold curves represent mean performance in hold-out testing of 1/5 of the data each time, with separate tests shown as dashed curves and with error bars showing +− 1SD. Metformin-treated T2D samples can be well separated from controls (using Intestinibacter abundance as the only feature), whereas distinguishing T2D metformin-samples from ND CTRL samples works poorly even in the best case, requiring 63 distinct microbial features to achieve this separation.
Figure 2. Gut microbiome signatures in metformin-naïve type 2 diabetes and in type 1 diabetes
Differences between healthy controls and T2D patients contrasted against T1D as an alternative form of dysglycaemia. A. Taxonomic and functional microbiome signatures of metformin-naïve type 2 diabetes. The heatmaps show bacterial genera (horizontal axis) and microbial gene functions (vertical axis) that are significantly (study source adjusted KW-test and post-hoc MWU test, markers in innermost marginal heatmaps indicating *: FDR<0.05; +: FDR<0.1) different in abundance (nonparametric enrichment scores shown as intensity of innermost marginal heatmaps; red-green colour scale) between T2D metformin− (n=106) and ND CTRL (n=554) gut metagenomes, revealing a robust diabetic signature across datasets. None of these features are significantly different in a comparison of T1D (n=31) with ND CTRL (n=277) gut metagenomes (outermost marginal heatmaps, same notation as above), implying they are not direct effects of dysglycaemia. The central heatmap shows Spearman correlations (purple to red color scale) between abundance of bacterial taxa and microbial gene modules (Spearman test FDR scores shown as markers *: FDR<0.05; ***: FDR<0.001). B. Elevated gene richness in adult type 1 diabetes samples. Comparing MHD samples only, T1D (n=31) gut metagenomes show significantly (MWU test, +: FDR<0.1, *: FDR<0.05) higher gut microbiome richness (i.e. gene count) than all other sample subsets (ND CTRL n=277, T2D metformin+ n=58, T2D metformin− n=17 gut metagenomes). Sample median richness is shown as horizontal black bars.
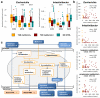
Figure 3. Impact of metformin on the human gut microbiome
Characterization of the microbially-mediated therapeutic and adverse effects of metformin. A. Gut microbial shifts under metformin treatment. Metformin treatment significantly (study-source adjusted KW-test and post-hoc MWU test, +: FDR<0.1; *: FDR<0.05; ***: FDR<0.001) increases Escherichia and lowers Intestinibacter abundance. Boxplots show median/quartile abundances, whiskers extend to 1.58 × interquartile range / sqrt (n), for T2D metformin+ (nCHN=15, nMHD=58, nSWE=20), T2D metformin− (nCHN=56, nMHD=17, nSWE=33) and ND CTRL (nCHN=185, nMHD=277, nSWE=92) gut metagenome samples. B. Correlations between serum levels of metformin and gut microbiota in Danish MetaHIT samples, including SCFA production modules. Serum metformin levels of T2D patients (n=75 gut metagenomes) are significantly (Spearman FDR < 0.1) positively correlated with Escherichia abundance, and in significant negative correlation with Intestinibacter abundance. Bacterial gene function modules for butyrate and propionate production increase in abundance as serum metformin levels increase. Dot markers are shown for all MHD samples where serum metformin concentration was measured. Metformin-untreated T2D samples (serum concentrations < 10 mg/ml) are shown in orange, treated samples in dark red. Spearman coefficients (calculated for treated samples only) and FDRs are shown. C. Microbial shifts under metformin treatment contribute to improved glucose control and to adverse effects. Schematic illustration of gut microbial changes and their impact on host health. Observed associations (orange lines) between microbial taxa abundances (orange ellipses), microbial functional potential (orange boxes), and blood values (filled orange boxes) and metformin treatment are linked with literature-derived metformin− or microbiota-induced host physiological effects (blue boxes and arrows; dashed arrows indicate hypothesized causality). Drug-host-microbiota interactions can contribute to previously described therapeutic (green triangles) and side (red triangles) effects of metformin treatment.
Comment in
- Gut microbiota: Antidiabetic drug treatment confounds gut dysbiosis associated with type 2 diabetes mellitus.
Holmes D. Holmes D. Nat Rev Endocrinol. 2016 Feb;12(2):61. doi: 10.1038/nrendo.2015.222. Epub 2015 Dec 15. Nat Rev Endocrinol. 2016. PMID: 26668122 No abstract available. - Metformin Joins Forces with Microbes.
Cabreiro F. Cabreiro F. Cell Host Microbe. 2016 Jan 13;19(1):1-3. doi: 10.1016/j.chom.2015.12.012. Cell Host Microbe. 2016. PMID: 26764588 - Confounding Effects of Metformin on the Human Gut Microbiome in Type 2 Diabetes.
Mardinoglu A, Boren J, Smith U. Mardinoglu A, et al. Cell Metab. 2016 Jan 12;23(1):10-2. doi: 10.1016/j.cmet.2015.12.012. Cell Metab. 2016. PMID: 26771114 - MICROBIOME. Prescription drugs obscure microbiome analyses.
Devkota S. Devkota S. Science. 2016 Jan 29;351(6272):452-3. doi: 10.1126/science.aaf1353. Science. 2016. PMID: 26823414 No abstract available.
Similar articles
- Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut.
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. de la Cuesta-Zuluaga J, et al. Diabetes Care. 2017 Jan;40(1):54-62. doi: 10.2337/dc16-1324. Epub 2016 Nov 14. Diabetes Care. 2017. PMID: 27999002 - Confounding Effects of Metformin on the Human Gut Microbiome in Type 2 Diabetes.
Mardinoglu A, Boren J, Smith U. Mardinoglu A, et al. Cell Metab. 2016 Jan 12;23(1):10-2. doi: 10.1016/j.cmet.2015.12.012. Cell Metab. 2016. PMID: 26771114 - Metformin and gut microbiota: their interactions and their impact on diabetes.
Vallianou NG, Stratigou T, Tsagarakis S. Vallianou NG, et al. Hormones (Athens). 2019 Jun;18(2):141-144. doi: 10.1007/s42000-019-00093-w. Epub 2019 Feb 4. Hormones (Athens). 2019. PMID: 30719628 Review. - Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India.
Alvarez-Silva C, Kashani A, Hansen TH, Pinna NK, Anjana RM, Dutta A, Saxena S, Støy J, Kampmann U, Nielsen T, Jørgensen T, Gnanaprakash V, Gnanavadivel R, Sukumaran A, Rani CSS, Færch K, Radha V, Balasubramanyam M, Nair GB, Das B, Vestergaard H, Hansen T, Mande SS, Mohan V, Arumugam M, Pedersen O. Alvarez-Silva C, et al. Genome Med. 2021 Mar 3;13(1):37. doi: 10.1186/s13073-021-00856-4. Genome Med. 2021. PMID: 33658058 Free PMC article. - Metformin: old friend, new ways of action-implication of the gut microbiome?
Rodriguez J, Hiel S, Delzenne NM. Rodriguez J, et al. Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):294-301. doi: 10.1097/MCO.0000000000000468. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29634493 Review.
Cited by
- Metformin modulates the gut microbiome in broiler breeder hens.
Van Syoc E, Weaver E, Rogers CJ, Silverman JD, Ramachandran R, Ganda E. Van Syoc E, et al. Front Physiol. 2022 Sep 14;13:1000144. doi: 10.3389/fphys.2022.1000144. eCollection 2022. Front Physiol. 2022. PMID: 36203937 Free PMC article. - Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss.
Day EA, Ford RJ, Smith BK, Mohammadi-Shemirani P, Morrow MR, Gutgesell RM, Lu R, Raphenya AR, Kabiri M, McArthur AG, McInnes N, Hess S, Paré G, Gerstein HC, Steinberg GR. Day EA, et al. Nat Metab. 2019 Dec;1(12):1202-1208. doi: 10.1038/s42255-019-0146-4. Epub 2019 Dec 9. Nat Metab. 2019. PMID: 32694673 - Antibiotic use and its consequences for the normal microbiome.
Blaser MJ. Blaser MJ. Science. 2016 Apr 29;352(6285):544-5. doi: 10.1126/science.aad9358. Science. 2016. PMID: 27126037 Free PMC article. Review. - Unique Pakistani gut microbiota highlights population-specific microbiota signatures of type 2 diabetes mellitus.
Saleem A, Ikram A, Dikareva E, Lahtinen E, Matharu D, Pajari AM, de Vos WM, Hasan F, Salonen A, Jian C. Saleem A, et al. Gut Microbes. 2022 Jan-Dec;14(1):2142009. doi: 10.1080/19490976.2022.2142009. Gut Microbes. 2022. PMID: 36322821 Free PMC article. - Rebalancing glucolipid metabolism and gut microbiome dysbiosis by nitrate-dependent alleviation of high-fat diet-induced obesity.
Ma L, Hu L, Jin L, Wang J, Li X, Wang W, Chang S, Zhang C, Wang J, Wang S. Ma L, et al. BMJ Open Diabetes Res Care. 2020 Aug;8(1):e001255. doi: 10.1136/bmjdrc-2020-001255. BMJ Open Diabetes Res Care. 2020. PMID: 32843498 Free PMC article.
References
- Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi:10.1038/nature11450. - PubMed
- Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi:10.1038/nature12198. - PubMed
- Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543–551. doi:10.7326/0003-4819-159-8-201310150-00007. - PubMed
Methods references
- Jorgensen T, et al. A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil. 2003;10:377–386. doi:10.1097/01.hjr.0000096541.30533.82. - PubMed
- WHO . Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. World Health Organization; Geneva: 1999. ( Tech. Rep. Ser. WHO/NCD/NCS/99.2 ed ).
- Li J, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–841. doi:10.1038/nbt.2942. - PubMed
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi:10.1093/bioinformatics/btl158. - PubMed
Extended Data references
- Abeel T, Helleputte T, Van de Peer Y, Dupont P, Saeys Y. Robust biomarker identification for cancer diagnosis with ensemble feature selection methods. Bioinformatics. 2010;26:392–398. doi:DOI 10.1093/bioinformatics/btp630. - PubMed
- Hildebrand F, et al. A comparative analysis of the intestinal metagenomes present in guinea pigs (Cavia porcellus) and humans (Homo sapiens) BMC Genomics. 2012;13(1):514. http://doi.org/10.1186/1471-2164-13-514. - DOI - PMC - PubMed
- Hildebrand F, et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biology. 2013;14(1):R4. http://doi.org/10.1186/gb-2013-14-1-r4. - DOI - PMC - PubMed
- Haraldsdóttir J, et al. Portionsstorleker - Nordiska standardportioner av mat och livsmedel (Portion sizes-Nordic standard portions of food and foodstuffs) Nordic Council & Council of Ministers; Sweden: 1998. pp. 1–41. 1998.
- Biltoft-Jensen A, et al. Danskernes kostvaner 2000-2002. Teknisk rapport. The National Dietary Survey of Denmark 2000-2002; 2005. Technical Report.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical