Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders - PubMed (original) (raw)
Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders
Soojeong Kang et al. J Biol Chem. 2016.
Abstract
Dysregulation of endoplasmic reticulum (ER) Ca(2+) homeostasis triggers ER stress leading to the development of insulin resistance in obesity and diabetes. Impaired function of the sarco/endoplasmic reticulum Ca(2+)-ATPase (SERCA) has emerged as a major contributor to ER stress. We pharmacologically activated SERCA2b in a genetic model of insulin resistance and type 2 diabetes (ob/ob mice) with a novel allosteric activator, CDN1163, which markedly lowered fasting blood glucose, improved glucose tolerance, and ameliorated hepatosteatosis but did not alter glucose levels or body weight in lean controls. Importantly, CDN1163-treated ob/ob mice maintained euglycemia comparable with that of lean mice for >6 weeks after cessation of CDN1163 administration. CDN1163-treated ob/ob mice showed a significant reduction in adipose tissue weight with no change in lean mass, assessed by magnetic resonance imaging. They also showed an increase in energy expenditure using indirect calorimetry, which was accompanied by increased expression of uncoupling protein 1 (UCP1) and UCP3 in brown adipose tissue. CDN1163 treatment significantly reduced the hepatic expression of genes involved in gluconeogenesis and lipogenesis, attenuated ER stress response and ER stress-induced apoptosis, and improved mitochondrial biogenesis, possibly through SERCA2-mediated activation of AMP-activated protein kinase pathway. The findings suggest that SERCA2b activation may hold promise as an effective therapy for type-2 diabetes and metabolic dysfunction.
Keywords: AMP-activated kinase (AMPK); Ca2+ homeostasis; SERCA2b; diabetes; endoplasmic reticulum stress (ER stress); glucose metabolism; hepatosteatosis; insulin sensitivity; lipid metabolism; mitochondria efficiency.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
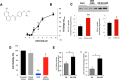
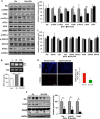
FIGURE 1.
CDN1163 is an allosteric activator of SERCA2b and enhances Ca2+ transport activity in the liver of obese mouse. A, chemical structure of CDN1163 SERCA activator (patents on file). B, the Ca2+-ATPase _V_max (limiting activity at 10 μ
m
Ca2+) was measured in ER vesicles from liver tissue after 20 min of incubation in the presence of various concentrations of CDN1163 as indicated (n = 6). C, effect of CDN1163 on ER Ca2+ accumulation in HEK cells that were either transfected with an adenovirus encoding Serca2b (Ad.Serca2b, multiplicity of infection 50) versus control or treated with CDN1163 (10 μ
m
) versus vehicle from a minimum of three determinations. A representative of Western blot of Serca2b expression is shown. *, p < 0.05; and **, p < 0.01 versus control. D, CDN1163 rescues cells from ER stress-induced cell death. ER stress-induced cell death was assessed in HEK cells either untreated or treated with vehicle (DMSO) or with hydrogen peroxide (H2O2) in the presence or absence of 10 μ
m
CDN1163 using CellTiter-Glo® luminescent cell viability assay. The data are expressed as the mean ± S.E. from at least three determinations. *, p < 0.05 versus H2O2; **, p < 0.01_versus_ vehicle. E, SERCA2b Ca2+ transport activity in vivo. Ca2+-ATPase activity (left) and the rate of Ca2+ uptake (right) were determined in liver ER microsomes purified from vehicle-treated obese (Ob) or CDN1163-treated obese mice (Ob+CDN) after treatment with 50 mg/kg CDN1163 for 5 days (n = 5 animals/group). Data are expressed as the means ± S.E. #, p < 0.05, obese versus CDN1163-treated obese mice.
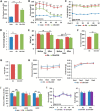
FIGURE 2.
CDN1163 improves glucose homeostasis and metabolic parameters in ob/ob mice. CDN1163 was administrated for 5 consecutive days (D0 to D4). A, fasting blood glucose levels after first injection of CDN1163 at day 1 (D1). Fasting blood glucose levels (B) and body weight (C) were determined weekly until day 50. D, average food intake in lean, vehicle-treated obese (Ob) and CDN1163-treated obese (Ob+CDN) mice (n = 10/group). E, body composition analyzed for 7 days in a separate cohort (n = 8) before and after 5 days of CDN1163 treatment in vehicle-treated obese (Ob) and CDN1163-treated obese (Ob+CDN) mice. Indirect calorimetry conducted for the last 4 days was assessed oxygen consumption (F), respiratory exchange ratio (RER) (G), and physical activity and caloric intake (H). J, mRNA expression of UCP1, UCP2, and UCP3 in brown tissue in lean, vehicle-treated obese (Ob) and CDN1163-treated obese (Ob+CDN) mice (n = 5). I, glucose levels and body weight after CDN1163 treatment of lean mice. CDN1163 was administrated for 5 consecutive days (D0 to D4). Fasting blood glucose levels and body weight were determined weekly until day 30 in lean plus vehicle (LN+Veh) and lean plus treatment (LN+CDN) mice (n = 6/group). Body weight values for day 1 (D1) and day 31 (D31) are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001, lean versus obese; #, p < 0.05; ##, p < 0.01; ###, p < 0.001, obese versus CDN1163-treated obese mice.
FIGURE 3.
CDN1163 increases glucose tolerance in ob/ob mice. A, intraperitoneal glucose tolerance test (GTT) assessed on day 8 in lean, vehicle-treated obese (Ob) and CDN1163-treated obese (Ob+CDN) mice (n = 10/group); glucose was measured at times shown after 1 g/kg of glucose injection and calculated as the area under the curve (AUC). B, the intraperitoneal insulin tolerance test was assessed on day 11 after 1 IU/kg of insulin injection 2 h after CDN injection, and glucose clearance is expressed as % reduction from basal levels, and (B) plasma insulin levels at end of study (day 50) are shown for lean and vehicle- and CDN1163-treated obese mice (n = 10) (C). D, representative from at least three experiments of Western blot analysis of insulin-stimulated Akt phosphorylation at serine 473 (pS-Akt) and threonine 308 (pT-Akt) in the liver after an acute insulin bolus (36.3 μg/ml in 0.9% saline (1 units/kg, assuming potency of 27.5 units/mg)). Protein loading was verified with total Akt and GAPDH. Also shown is Western blot liver SERCA2b expression in the three different groups of mice with GAPDH as a loading control. E, quantitative real-time PCR analysis of genes involved in gluconeogenesis in the liver in vehicle-treated obese (Ob) and CDN1163-treated (Ob+CDN) mice. Data are expressed as the means ± S.E. from at least 3–5 determinations. *, p < 0.05; **, p < 0.01; ***, p < 0.001, lean versus obese; #, p < 0.05 and ###, p < 0.001, obese versus CDN1163-treated obese mice. Data in C–E were performed at the end of the study (i.e. day 50). G6Pase, glucose-6-phosphatase.
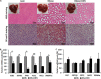
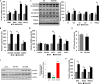
FIGURE 4.
CDN1163 reduces lipid accumulation and decreases lipogenesis in obese mice livers. A, lipid accumulation measured with H&E (top) and Oil red O staining (bottom) in the liver of lean, vehicle-treated obese (Obese) and CDN1163-treated obese (Obese+CDN) mice (n = 5). qRT-PCR analysis of genes involved in liver de novo lipogenesis (B) and lipid oxidation (C) in obese (Ob) versus obese plus CDN1163 (Ob+CDN) mice. Bar, 50 μm. Data are expressed as the means ± S.E. (n = 5). #, p < 0.05; ##, p < 0.01; ###, p < 0.001, obese versus CDN1163-treated obese mice. All determinations were performed on harvested liver tissues at the end of the study (i.e. day 50).
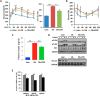
FIGURE 5.
CDN1163 relieves ER stress and attenuates apoptosis in the liver of ob/ob mice. Shown are Western blot analyses and densitometry quantification of ER stress markers (A) and qRT-PCR analyses of ER chaperones in vehicle-treated obese (Ob) versus CDN1163-treated obese (Ob+CDN) mice (C). B, determination and quantification of spliced (s) and unspliced (u) XBP1 in lean (L), vehicle-treated obese (O), and CDN1163-treated obese (OC) mice. D, TUNEL staining of apoptotic cells in liver tissues from vehicle-treated obese (ob/_ob_-vehicle) versus CDN1163-treated obese (ob/_ob_-CDN1163) mice (bar = 50 μm) and quantification of apoptosis shown as a percentage of apoptotic nuclei (red by TUNEL) versus total nuclei (blue by DAPI) (n = 5). E, representative Western blot analyses and quantification of apoptosis markers in liver tissues from vehicle-treated obese (Ob) versus CDN1163-treated obese (Ob+CDN) mice. Data are expressed as the means ± S.E. from at least 3–5 determinations. **, p < 0.01, lean versus obese; #, p < 0.05, ##, p < 0.01, and *, p < 0.05, obese versus CDN1163-treated obese mice. All determinations were performed on harvested liver tissues at the end of the study (i.e. day 50).
FIGURE 6.
CDN1163 improves mitochondrial efficiency in the liver of ob/ob mice. All determinations were performed on harvested liver tissues at the end of the study (i.e. day 50). qRT-PCR analyses of genes involved in mitochondrial biosynthesis (A) and mitochondrial DNA contents (B). C, Western blot analyses and quantification of proteins involved in OXPHOS and qRT-PCR analyses of OXPHOS genes (D), ATP content (E), and antioxidant enzymes (G) in vehicle (Ob)- versus CDN1163-treated obese (Ob+CDN) mice. F, AMPK phosphorylation (pAMPK) and densitometry (normalized to total AMPK (tAMPK)) in liver samples from lean, vehicle-treated (Ob) versus CDN1163-treated obese (Ob-CDN) mice. GAPDH is a loading control. Data are expressed as the means ± S.E. from at least three experiments; #, p < 0.05 and ##, p < 0.01 obese versus CDN-treated obese mice; **, p < 0.01 versus lean and ***, p < 0.001 CDN1163 versus vehicle.
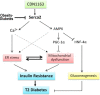
FIGURE 7.
Schematic diagram depicting proposed molecular mechanism underlying CDN1163/SERCA2b metabolic benefits. A fundamental abnormality of obesity and diabetes is down-regulation and dysfunction of Serca2b causing intracellular Ca2+ imbalance with a concomitant induction of ER stress and mitochondrial dysfunction, as these two events are reciprocally related. ER stress and mitochondrial dysfunction then trigger insulin resistance and development of diabetes. Pharmacological restoration of Serca2b activity by CDN1163 (a) normalizes intracellular Ca2+ dyshomeostasis (which reestablishes ER homeostasis) and (b) activates AMPK, which in turn up-regulates PGC1α (which improves mitochondrial biogenesis) and down-regulates HNF4α leading to suppression of gluconeogenesis. Evidence also shows that AMPK can improve ER stress and Ca2+ and has long played a major role in mitochondria (dashed lines). Attenuation of gluconeogenesis and ER stress and improvement of mitochondrial efficiency altogether then ameliorate insulin resistance and diabetes.
Similar articles
- CDN1163, an activator of sarco/endoplasmic reticulum Ca2+ ATPase, up-regulates mitochondrial functions and protects against lipotoxicity in pancreatic β-cells.
Nguyen HT, Noriega Polo C, Wiederkehr A, Wollheim CB, Park KS. Nguyen HT, et al. Br J Pharmacol. 2023 Nov;180(21):2762-2776. doi: 10.1111/bph.16160. Epub 2023 Jul 11. Br J Pharmacol. 2023. PMID: 37277321 - A natural compound jaceosidin ameliorates endoplasmic reticulum stress and insulin resistance via upregulation of SERCA2b.
Ouyang Z, Li W, Meng Q, Zhang Q, Wang X, Elgehama A, Wu X, Shen Y, Sun Y, Wu X, Xu Q. Ouyang Z, et al. Biomed Pharmacother. 2017 May;89:1286-1296. doi: 10.1016/j.biopha.2017.03.023. Epub 2017 Mar 17. Biomed Pharmacother. 2017. PMID: 28320096 - Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity.
Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Park SW, et al. Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19320-5. doi: 10.1073/pnas.1012044107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974941 Free PMC article. - Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity.
Villalobos-Labra R, Subiabre M, Toledo F, Pardo F, Sobrevia L. Villalobos-Labra R, et al. Mol Aspects Med. 2019 Apr;66:49-61. doi: 10.1016/j.mam.2018.11.001. Epub 2018 Nov 27. Mol Aspects Med. 2019. PMID: 30472165 Review. - SERCA stimulation: A potential approach in therapeutics.
Rahate K, Bhatt LK, Prabhavalkar KS. Rahate K, et al. Chem Biol Drug Des. 2020 Jan;95(1):5-15. doi: 10.1111/cbdd.13620. Epub 2019 Sep 30. Chem Biol Drug Des. 2020. PMID: 31512386 Review.
Cited by
- A target-agnostic screen identifies approved drugs to stabilize the endoplasmic reticulum-resident proteome.
Henderson MJ, Trychta KA, Yang SM, Bäck S, Yasgar A, Wires ES, Danchik C, Yan X, Yano H, Shi L, Wu KJ, Wang AQ, Tao D, Zahoránszky-Kőhalmi G, Hu X, Xu X, Maloney D, Zakharov AV, Rai G, Urano F, Airavaara M, Gavrilova O, Jadhav A, Wang Y, Simeonov A, Harvey BK. Henderson MJ, et al. Cell Rep. 2021 Apr 27;35(4):109040. doi: 10.1016/j.celrep.2021.109040. Cell Rep. 2021. PMID: 33910017 Free PMC article. - Cardioprotective effects of GPER agonist in ovariectomized diabetic rats: reversing ER stress and structural changes.
Sirizi MAG, Esmailidehaj M, Mohamadi-Zarch SM, Yadeghari M, Azizian H. Sirizi MAG, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep 19. doi: 10.1007/s00210-024-03438-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39298018 - Deletion of Tmtc4 activates the unfolded protein response and causes postnatal hearing loss.
Li J, Akil O, Rouse SL, McLaughlin CW, Matthews IR, Lustig LR, Chan DK, Sherr EH. Li J, et al. J Clin Invest. 2018 Nov 1;128(11):5150-5162. doi: 10.1172/JCI97498. Epub 2018 Oct 15. J Clin Invest. 2018. PMID: 30188326 Free PMC article. - Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation.
Papp B, Launay S, Gélébart P, Arbabian A, Enyedi A, Brouland JP, Carosella ED, Adle-Biassette H. Papp B, et al. Int J Mol Sci. 2020 May 9;21(9):3351. doi: 10.3390/ijms21093351. Int J Mol Sci. 2020. PMID: 32397400 Free PMC article. Review. - Phenolic Compounds from Morus nigra Regulate Viability and Apoptosis of Pancreatic β-Cells Possibly via SERCA Activity.
Heger V, Benesova B, Viskupicova J, Majekova M, Zoofishan Z, Hunyadi A, Horakova L. Heger V, et al. ACS Med Chem Lett. 2020 Mar 26;11(5):1006-1013. doi: 10.1021/acsmedchemlett.0c00047. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435418 Free PMC article.
References
- Disdier-Flores O. M., Rodríguez-Lugo L. A., Pérez-Perdomo R., and Pérez-Cardona C. M. (2001) The public health burden of diabetes: a comprehensive review. P. R. Health Sci. J. 20, 123–130 - PubMed
- Williams L. M. (2012) Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 71, 521–533 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK099463/DK/NIDDK NIH HHS/United States
- R01 HL097357/HL/NHLBI NIH HHS/United States
- R01 AA023416/AA/NIAAA NIH HHS/United States
- DK020541/DK/NIDDK NIH HHS/United States
- HL097375/HL/NHLBI NIH HHS/United States
- R01 HL128072/HL/NHLBI NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- DK083658/DK/NIDDK NIH HHS/United States
- R01 DK083658/DK/NIDDK NIH HHS/United States
- R01 HL129814/HL/NHLBI NIH HHS/United States
- R01 DK111417/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- R56 DK083658/DK/NIDDK NIH HHS/United States
- R56 DK100624/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous