Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise-Induced Muscle Protein Anabolism - PubMed (original) (raw)
Review
Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise-Induced Muscle Protein Anabolism
Paul T Reidy et al. J Nutr. 2016 Feb.
Abstract
The goal of this critical review is to comprehensively assess the evidence for the molecular, physiologic, and phenotypic skeletal muscle responses to resistance exercise (RE) combined with the nutritional intervention of protein and/or amino acid (AA) ingestion in young adults. We gathered the literature regarding the translational response in human skeletal muscle to acute exposure to RE and protein/AA supplements and the literature describing the phenotypic skeletal muscle adaptation to RE and nutritional interventions. Supplementation of protein/AAs with RE exhibited clear protein dose-dependent effects on translational regulation (protein synthesis) through mammalian target of rapamycin complex 1 (mTORC1) signaling, which was most apparent through increases in p70 ribosomal protein S6 kinase 1 (S6K1) phosphorylation, compared with postexercise recovery in the fasted or carbohydrate-fed state. These acute findings were critically tested via long-term exposure to RE training (RET) and protein/AA supplementation, and it was determined that a diminishing protein/AA supplement effect occurs over a prolonged exposure stimulus after exercise training. Furthermore, we found that protein/AA supplements, combined with RET, produced a positive, albeit minor, effect on the promotion of lean mass growth (when assessed in >20 participants/treatment); a negligible effect on muscle mass; and a negligible to no additional effect on strength. A potential concern we discovered was that the majority of the exercise training studies were underpowered in their ability to discern effects of protein/AA supplementation. Regardless, even when using optimal methodology and large sample sizes, it is clear that the effect size for protein/AA supplementation is low and likely limited to a subset of individuals because the individual variability is high. With regard to nutritional intakes, total protein intake per day, rather than protein timing or quality, appears to be more of a factor on this effect during long-term exercise interventions. There were no differences in strength or mass/muscle mass on RET outcomes between protein types when a leucine threshold (>2 g/dose) was reached. Future research with larger sample sizes and more homogeneity in design is necessary to understand the underlying adaptations and to better evaluate the individual variability in the muscle-adaptive response to protein/AA supplementation during RET.
Keywords: exercise training; leucine; mTORC1; protein synthesis; skeletal muscle.
© 2016 American Society for Nutrition.
Conflict of interest statement
Author disclosures: PT Reidy and BB Rasmussen, no conflicts of interest.
Figures
FIGURE 1
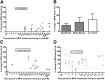
Effect of protein/AA supplementation on postexercise MPS in young adults. Percentage changes from fasted to protein/AA supplemented states on MPS via the direct precursor product method (either the myofibrillar or mixed-muscle protein fractions) and arterial and venous balance methods (2- and 3-pool models) plotted from individual studies according to the time period (h) of assessment post-RE. Studies with an ∼900% response during exercise (115) and ∼600% responses at 1–2 and 3–4 h (53) were removed from panel D to shorten the y axis. Each data point represents a mean response value from a treatment arm in a clinical trial: n = 30 for myofibrillar (A), n = 16 for mixed muscle (C), and n = 21 for 2- and 3-pool models (D) treatment arms. The horizontal (dashed or dotted) line in each column represents the mean response for all treatments in that time period. Panel B shows the mean (95% CI) pooled treatment responses over all time periods. AA, amino acid; EX, exercise; MPS, muscle protein synthesis; PLA, placebo; RE, resistance exercise.
FIGURE 2
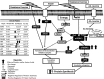
Representative schematic for the effect of postexercise PRO/AA supplementation on the overall mTORC1 signaling and MPS response in human skeletal muscle. AA, amino acid; Akt, protein kinase B; AMPK, AMP-activated protein kinase; eEF2, eukaryotic elongation factor 2; eEF2K, eukaryotic elongation factor 2 kinase; eIF2B, eukaryotic initiation factor 2B; ERK1/2, extracellular-related kinase 1/2; Gator, GTPase-activating protein activity toward Rags; MPS, muscle protein synthesis; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PA, phosphatidic acid; Phosph, phosphorylation; PRAS40, proline-rich Akt substrate 40; PRO, protein; Rags, recombination activating genes; Raptor, regulatory-associated protein of mTOR; RE, resistance exercise; Rheb, Ras homolog enriched in brain; rpS6, ribosomal protein S6; S6K1/p70S6K1, p70 ribosomal protein S6 kinase 1; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2; 4E-BP1, eukaryotic initiation factor 4E binding protein 1.
FIGURE 3
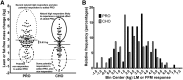
Effect of protein supplementation during resistance exercise training on the individual response for LM or FFM changes. These data were extracted from diverse clinical trials reporting the scatterplot (A) of the individual change after supplementation with 20–30 g protein (n = 95) combining the similar overall average changes in a protein blend (PT Reidy and BB Rasmussen, unpublished data), milk protein (94, 196), and whey protein (; PT Reidy and BB Rasmussen, unpublished data) compared with isocaloric maltodextrin carbohydrate (n = 57) (, ; PT Reidy and BB Rasmussen, unpublished data). The solid lines in panel A represent the means (95% CIs) of each pooled group (PRO and CHO). Hartman et al. (196) used FFM. The data were pooled into a relative frequency histogram (B). CHO, carbohydrate; FFM, fat-free mass; LM, lean mass; PRO, protein.
Similar articles
- Effect of dietary arachidonic acid supplementation on acute muscle adaptive responses to resistance exercise in trained men: a randomized controlled trial.
Mitchell CJ, D'Souza RF, Figueiredo VC, Chan A, Aasen K, Durainayagam B, Mitchell S, Sinclair AJ, Egner IM, Raastad T, Cameron-Smith D, Markworth JF. Mitchell CJ, et al. J Appl Physiol (1985). 2018 Apr 1;124(4):1080-1091. doi: 10.1152/japplphysiol.01100.2017. Epub 2018 Feb 1. J Appl Physiol (1985). 2018. PMID: 29389245 Clinical Trial. - Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans.
Churchward-Venne TA, Murphy CH, Longland TM, Phillips SM. Churchward-Venne TA, et al. Amino Acids. 2013 Aug;45(2):231-40. doi: 10.1007/s00726-013-1506-0. Epub 2013 May 5. Amino Acids. 2013. PMID: 23645387 - Presleep protein ingestion does not compromise the muscle protein synthetic response to protein ingested the following morning.
Wall BT, Burd NA, Franssen R, Gorissen SH, Snijders T, Senden JM, Gijsen AP, van Loon LJ. Wall BT, et al. Am J Physiol Endocrinol Metab. 2016 Dec 1;311(6):E964-E973. doi: 10.1152/ajpendo.00325.2016. Epub 2016 Oct 25. Am J Physiol Endocrinol Metab. 2016. PMID: 27780822 - Synergistic effects of resistance training and protein intake: practical aspects.
Guimarães-Ferreira L, Cholewa JM, Naimo MA, Zhi XI, Magagnin D, de Sá RB, Streck EL, Teixeira Tda S, Zanchi NE. Guimarães-Ferreira L, et al. Nutrition. 2014 Oct;30(10):1097-103. doi: 10.1016/j.nut.2013.12.017. Epub 2014 Jan 10. Nutrition. 2014. PMID: 24751198 Review. - Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: role of the MPS response.
Cholewa JM, Dardevet D, Lima-Soares F, de Araújo Pessôa K, Oliveira PH, Dos Santos Pinho JR, Nicastro H, Xia Z, Cabido CE, Zanchi NE. Cholewa JM, et al. Amino Acids. 2017 May;49(5):811-820. doi: 10.1007/s00726-017-2390-9. Epub 2017 Feb 7. Amino Acids. 2017. PMID: 28175999 Review.
Cited by
- Effects of Leucine-Enriched Whey Protein Supplementation on Physical Function in Post-Hospitalized Older Adults Participating in 12-Weeks of Resistance Training Program: A Randomized Controlled Trial.
Amasene M, Besga A, Echeverria I, Urquiza M, Ruiz JR, Rodriguez-Larrad A, Aldamiz M, Anaut P, Irazusta J, Labayen I. Amasene M, et al. Nutrients. 2019 Oct 1;11(10):2337. doi: 10.3390/nu11102337. Nutrients. 2019. PMID: 31581591 Free PMC article. Clinical Trial. - High-intensity leg cycling alters the molecular response to resistance exercise in the arm muscles.
Moberg M, Apró W, Cervenka I, Ekblom B, van Hall G, Holmberg HC, Ruas JL, Blomstrand E. Moberg M, et al. Sci Rep. 2021 Mar 19;11(1):6453. doi: 10.1038/s41598-021-85733-1. Sci Rep. 2021. PMID: 33742064 Free PMC article. Clinical Trial. - Protein Supplementation Has Minimal Effects on Muscle Adaptations during Resistance Exercise Training in Young Men: A Double-Blind Randomized Clinical Trial.
Reidy PT, Borack MS, Markofski MM, Dickinson JM, Deer RR, Husaini SH, Walker DK, Igbinigie S, Robertson SM, Cope MB, Mukherjea R, Hall-Porter JM, Jennings K, Volpi E, Rasmussen BB. Reidy PT, et al. J Nutr. 2016 Sep;146(9):1660-9. doi: 10.3945/jn.116.231803. Epub 2016 Jul 27. J Nutr. 2016. PMID: 27466602 Free PMC article. Clinical Trial. - Ketogenic Diet and Skeletal Muscle Hypertrophy: A Frenemy Relationship?
Paoli A, Cancellara P, Pompei P, Moro T. Paoli A, et al. J Hum Kinet. 2019 Aug 21;68:233-247. doi: 10.2478/hukin-2019-0071. eCollection 2019 Aug. J Hum Kinet. 2019. PMID: 31531148 Free PMC article. - The effects of acute aerobic and resistance exercise on mTOR signaling and autophagy markers in untrained human skeletal muscle.
Mazo CE, D'Lugos AC, Sweeney KR, Haus JM, Angadi SS, Carroll CC, Dickinson JM. Mazo CE, et al. Eur J Appl Physiol. 2021 Oct;121(10):2913-2924. doi: 10.1007/s00421-021-04758-6. Epub 2021 Jul 1. Eur J Appl Physiol. 2021. PMID: 34196787 Free PMC article.
References
- Kraemer WJ, Deschenes MR, Fleck SJ. Physiological adaptations to resistance exercise: implications for athletic conditioning. Sports Med 1988;6:246–56. - PubMed
- Abernethy PJ, Jurimae J, Logan PA, Taylor AW, Thayer RE. Acute and chronic response of skeletal muscle to resistance exercise. Sports Med 1994;17:22–38. - PubMed
- Matoba H, Gollnick PD. Response of skeletal muscle to training. Sports Med 1984;1:240–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical