Multivariate Calibration Approach for Quantitative Determination of Cell-Line Cross Contamination by Intact Cell Mass Spectrometry and Artificial Neural Networks - PubMed (original) (raw)
Multivariate Calibration Approach for Quantitative Determination of Cell-Line Cross Contamination by Intact Cell Mass Spectrometry and Artificial Neural Networks
Elisa Valletta et al. PLoS One. 2016.
Abstract
Cross-contamination of eukaryotic cell lines used in biomedical research represents a highly relevant problem. Analysis of repetitive DNA sequences, such as Short Tandem Repeats (STR), or Simple Sequence Repeats (SSR), is a widely accepted, simple, and commercially available technique to authenticate cell lines. However, it provides only qualitative information that depends on the extent of reference databases for interpretation. In this work, we developed and validated a rapid and routinely applicable method for evaluation of cell culture cross-contamination levels based on mass spectrometric fingerprints of intact mammalian cells coupled with artificial neural networks (ANNs). We used human embryonic stem cells (hESCs) contaminated by either mouse embryonic stem cells (mESCs) or mouse embryonic fibroblasts (MEFs) as a model. We determined the contamination level using a mass spectra database of known calibration mixtures that served as training input for an ANN. The ANN was then capable of correct quantification of the level of contamination of hESCs by mESCs or MEFs. We demonstrate that MS analysis, when linked to proper mathematical instruments, is a tangible tool for unraveling and quantifying heterogeneity in cell cultures. The analysis is applicable in routine scenarios for cell authentication and/or cell phenotyping in general.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
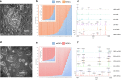
Fig 1
(A) Colony of human embryonic stem cells (hESCs) cultured on a feeder layer of mouse embryonic fibroblasts. (B) Experimental ratios of two-component mixtures of hESCs + MEFs. (C) Representative mass spectra of selected two-component mixtures of hESCs + MEFs. (D) Colony of mouse embryonic stem cells (mESCs) in a feeder-free culture. (E) Experimental ratios of two-component mixtures of hESCs + mESCs. (F) Representative mass spectra of selected two-component mixtures of hESCs + mESCs.
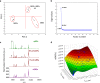
Fig 2
(A) Principal component analysis of mass spectra dataset containing intensities of 84 m/z for pure MEF and hESC populations and their 1:1 mixture. (B) Scree plot documenting the presence of three factors contributing predominantly to the overall variability in the analyzed dataset. (C) Pre-processed MALDI-TOF mass spectra for pure hESCs and MEFs and a hESCs + MEFs two-component mixture containing 99% hESCs and 1% MEFs. The spectra were normalized to vector of unit length (a.u.). Asterisks indicate peaks at m/z 3992 and 9908. (D) Surface plot of intensities of peaks at m/z 3992 and 9908 versus the number of MEFs in hESCs + MEFs two-component mixtures.
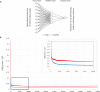
Fig 3
(A) Optimal ANN architecture (one Input layer, one Hidden layer with four neurons, and one Output layer). (B) Training and leave-one-out verification plot of the RMS versus the number of training cycles (epochs). First 50 000 iterations are shown. The inset shows a detailed plot for the first 10 000 training cycles.
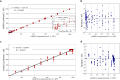
Fig 4
(A) Correlation between ANN-predicted number of cells and the experimental number of MEFs in two-component mixtures of hESCs + MEFs. The inset shows the correlation between experimental and predicted values in low concentration ranges of MEFs up to the 50×103 cells in the two-component mixtures. (B) Overview of Residuals (difference between ANN-predicted number of cells and the experimental values) versus the experimental number of MEF cells in two-component mixtures of hESCs + MEFs. (C) Correlation between ANN-predicted number of cells and the experimental number of mESCs in two-component mixtures of hESCs + mESCs. (D) Overview of Residuals (difference between ANN-predicted number of cells and the experimental values) versus the experimental number of mESC cells in the two-component mixtures of hESCs + mESCs.
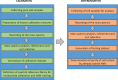
Fig 5. Overview for quantitative ANN-coupled MS-based analysis of cross-contamination.
Fig 6. Experimental schematic of the multivariate calibration-based ANN spectral analysis.
Similar articles
- Calibration in non-linear NIR spectroscopy using principal component artificial neural networks.
Dou Y, Zou T, Liu T, Qu N, Ren Y. Dou Y, et al. Spectrochim Acta A Mol Biomol Spectrosc. 2007 Dec 31;68(5):1201-6. doi: 10.1016/j.saa.2007.01.021. Epub 2007 Jan 30. Spectrochim Acta A Mol Biomol Spectrosc. 2007. PMID: 17336581 - Quantitative analysis of multivariate data using artificial neural networks: a tutorial review and applications to the deconvolution of pyrolysis mass spectra.
Goodacre R, Neal MJ, Kell DB. Goodacre R, et al. Zentralbl Bakteriol. 1996 Aug;284(4):516-39. doi: 10.1016/s0934-8840(96)80004-1. Zentralbl Bakteriol. 1996. PMID: 8899971 Review. - A nested multivariate chemometrics based calibration strategy for direct trace biometal analysis in soft tissue utilizing Energy Dispersive X-Ray Fluorescence (EDXRF) and scattering spectrometry.
Okonda JJ, Angeyo KH, Mangala JM, Kisia SM. Okonda JJ, et al. Appl Radiat Isot. 2017 Nov;129:49-56. doi: 10.1016/j.apradiso.2017.08.008. Epub 2017 Aug 8. Appl Radiat Isot. 2017. PMID: 28806597 Review.
Cited by
- The use of an artificial neural network to model the infection strategy for baculovirus production in suspended insect cell cultures.
Contreras-Gómez A, Beas-Catena A, Sánchez-Mirón A, García-Camacho F, Molina Grima E. Contreras-Gómez A, et al. Cytotechnology. 2018 Apr;70(2):555-565. doi: 10.1007/s10616-017-0128-x. Epub 2017 Aug 4. Cytotechnology. 2018. PMID: 28779292 Free PMC article. - Investigation of Cross-Contamination and Misidentification of 278 Widely Used Tumor Cell Lines.
Huang Y, Liu Y, Zheng C, Shen C. Huang Y, et al. PLoS One. 2017 Jan 20;12(1):e0170384. doi: 10.1371/journal.pone.0170384. eCollection 2017. PLoS One. 2017. PMID: 28107433 Free PMC article. - Intact Cell Mass Spectrometry as a Quality Control Tool for Revealing Minute Phenotypic Changes of Cultured Human Embryonic Stem Cells.
Vaňhara P, Kučera L, Prokeš L, Jurečková L, Peña-Méndez EM, Havel J, Hampl A. Vaňhara P, et al. Stem Cells Transl Med. 2018 Jan;7(1):109-114. doi: 10.1002/sctm.17-0107. Epub 2017 Dec 16. Stem Cells Transl Med. 2018. PMID: 29248004 Free PMC article. - Artificial Intelligence in Nutrients Science Research: A Review.
Sak J, Suchodolska M. Sak J, et al. Nutrients. 2021 Jan 22;13(2):322. doi: 10.3390/nu13020322. Nutrients. 2021. PMID: 33499405 Free PMC article. Review. - Cisplatin, glutathione and the third wheel: a copper-(1,10-phenanthroline) complex modulates cisplatin-GSH interactions from antagonism to synergism in cancer cells resistant to cisplatin.
Vascellari S, Valletta E, Perra D, Pinna E, Serra A, Isaia F, Pani A, Pivetta T. Vascellari S, et al. RSC Adv. 2019 Feb 12;9(10):5362-5376. doi: 10.1039/c8ra09652j. eCollection 2019 Feb 11. RSC Adv. 2019. PMID: 35515894 Free PMC article.
References
- Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RAF, et al. (2010) Check your cultures! A list of cross-contaminated or misidentified cell lines. International Journal of Cancer 127: 1–8. - PubMed
- Marx V (2014) Cell-line authentication demystified. Nature Methods 11: 483–+. - PubMed
- Nardone RM (2007) Eradication of cross-contaminated cell lines: A call for action. Cell Biology and Toxicology 23: 367–372. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources