Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: a mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle - PubMed (original) (raw)
Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: a mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle
Sandeep Balu Shelar et al. FASEB J. 2016 May.
Abstract
Recently we have reported that age-dependent decline in antioxidant levels accelerated apoptosis and skeletal muscle degeneration. Here, we demonstrate genetic ablation of the master cytoprotective transcription factor, nuclear factor (erythroid-derived-2)-like 2 (Nrf2), aggravates cardiotoxin (CTX)-induced tibialis anterior (TA) muscle damage. Disruption of Nrf2 signaling sustained the CTX-induced burden of reactive oxygen species together with compromised expression of antioxidant genes and proteins. Transcript/protein expression of phenotypic markers of muscle differentiation, namely paired box 7 (satellite cell) and early myogenic differentiation and terminal differentiation (myogenin and myosin heavy chain 2) were increased on d 2 and 4 postinjury but later returned to baseline levels on d 8 and 15 in wild-type (WT) mice. In contrast, these responses were persistently augmented in Nrf2-null mice suggesting that regulation of the regeneration-related signaling mechanisms require Nrf2 for normal functioning. Furthermore, Nrf2-null mice displayed slower regeneration marked by dysregulation of embryonic myosin heavy chain temporal expression. Histologic observations illustrated that Nrf2-null mice displayed smaller, immature TA muscle fibers compared with WT counterparts on d 15 after CTX injury. Improvement in TA muscle morphology and gain in muscle mass evident in the WT mice was not noticeable in the Nrf2-null animals. Taken together these data show that the satellite cell activation, proliferation, and differentiation requires a functional Nrf2 system for effective healing following injury.-Shelar, S. B., Narasimhan, M., Shanmugam, G., Litovsky, S. H., Gounder, S. S., Karan, G., Arulvasu, C., Kensler, T. W., Hoidal, J. R., Darley-Usmar, V. M., Rajasekaran, N. S. Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: a mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle.
Keywords: Nrf2/ARE; Pax7; TA muscle; cardiotoxin; satellite cell.
© FASEB.
Figures
Figure 1.
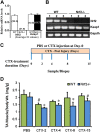
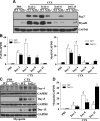
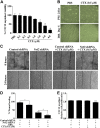
Model for CTX-induced skeletal muscle injury and regeneration. A, B) Confirmation of genotypes of WT and Nrf2−/− mice by analysis of relative mRNA levels of Nrf2 and Keap1 genes, analyzed by real-time quantitative PCR (A) and traditional PCR analysis (B) in TA muscle. C) Experimental strategy for CTX-mediated TA muscle injury and post-CTX injury regeneration of skeletal muscle regeneration. WT and Nrf2−/− test animals injected with 10 μM CTX in PBS into the TA muscle and control animals received equal volumes of PBS. D) Graphical presentation of means ±
sd
of TA muscle (mg)/body weight (g) of WT and Nrf2−/− mice to measure the CTX-mediated TA muscle damage and post-CTX injury regeneration at the indicated points. n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 2.
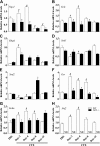
Down-regulation of Nrf2-regulated antioxidant gene expression in the CTX-injured regenerating TA muscles of Nrf2−/− mice. Transcript levels of Nrf2 (A), Gclc (B), Gclm (C), Nqo1 (D), G6pd (E), Gsr (F), Sod1 (G), and Sod2 (H) were determined in WT and Nrf2−/− TA muscle by quantitative RT-PCR. The relative gene expression was calculated by normalizing the mRNA levels of gene of interest with the levels of housekeeping gene, Gapdh. n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 3.
Poor recovery of Nrf2-regulated antioxidant protein levels in regenerating TA muscles of Nrf2−/− mice injured with CTX. A–D) Protein levels of Nrf2-regulated antioxidants (catalase, NQO1, GCLC, G6PD, SOD1, and SOD2) were determined by Western blot analysis of tissues homogenates of regenerating TA muscles collected after CTX-mediated injury and regeneration on d 2 (A), d 4 (B), d 8 (C), and d 15 (D). E) Western blots for GSR investigating changes in its levels during post-CTX injury regeneration of TA muscle of WT and Nrf2−/−. F–L) ImageJ densitometry quantification for NQO1 (F), GCLC (G), G6PD (H), SOD1 (I), SOD2 (J), GSR (K), and catalase (L) immunoblots. n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 4.
Nrf2-deficient mice displayed increased levels of ROS in CTX-injured regenerating TA muscle. A, B) Frozen TA muscle sections from control and CTX-treated WT and Nrf2−/− mice were loaded with fluorescent ROS probes: DHE (A) and chloromethyl derivative of 2′7′-dichlorodihydrofluorescein diacetate (CM-DCFDA) (B) were processed for fluorescent microscope (×40 magnification) analysis. C, D) The mean fluorescence intensity of DHE (red fluorescence) (C) and CM-DCFDA (green fluorescence) (D) were measured by ImageJ software; n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 5.
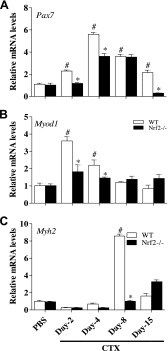
Delayed transcriptional activation of skeletal muscle regeneration markers in regenerating TA muscle of Nrf2−/− mice. Real-time quantitative PCR analyses of skeletal muscle regeneration markers Pax7 (A), Myod1 (B), and Myh2 (C) in TA muscle of PBS and CTX-treated WT and Nrf2−/− mice. Relative mRNA levels were calculated by normalizing to Gapdh expression. n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 6.
Loss of Nrf2 affects the expression of skeletal muscle regeneration markers during CTX-mediated TA muscle injury and regeneration. A, C) Immunoblot analyses for PAX7 and MYOD1 (A) and myogenin (C) in TA muscle of PBS and CTX-treated WT and Nrf2−/− mice. B, D) Relative intensity of the PAX7 and MYOD1 (B) and myogenin (D) protein signals normalized to GAPDH was calculated using ImageJ software; n = 4/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
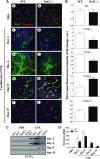
Figure 7.
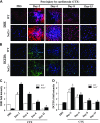
Absence of antioxidant defense hampers activation and proliferation of satellite cells in regenerating TA muscle. A) Immunofluorescence images showing Pax7+ satellite cells (green fluorescence) and the muscle cell plasma membrane protein laminin staining (red fluorescence) of TA muscles from WT (a, c, e, g, i) and Nrf2−/− (b, d, f, h, j) mice injected with PBS (a, b) and CTX (c_–_j) and collected on d 2 (c, d), 4 (e, f), 8 (g, h), and 15 (i, j) postinjury (×40 magnification). B) Graphs represent the mean fluorescence intensity of Pax7 expression. C) Western blot analysis of proliferation marker PCNA in the TA muscle of WT and Nrf2−/− mice. D) Densitometric analysis of PCNA expression was calculated using ImageJ software and the data are presented in a bar graph. n = 3/d per group. Statistical significance was calculated by 1-way ANOVA. #P < 0.05 PBS vs. CTX-WT, *P < 0.05 CTX-WT vs. Nrf2−/−.
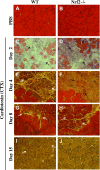
Figure 8.
Regeneration of CTX-induced TA muscle injury was delayed in Nrf2−/− mice. Bright field images of hematoxylin and eosin-stained cross sections of TA muscle injected with PBS (A, B) and post-CTX injury TA muscles collected on d 2 (C, D), d 4 (E, F), d 8 (G, H) and d 15 (I, J) of WT (A, C, E, G, I) and Nrf2−/− (B, D, F, H, J) mice (n = 4–5 mice/group). On d 2 postinjury, TA muscles of WT and Nrf2−/− mice show formation of edema and inflammatory infiltration (C, D) (black arrow). On d 4 (E, G) and d 8 postinjury (H), muscles show the presence of multinucleated myofibers (white arrow). On d 15 postinjury (I, J) larger cross-sectional and multinucleated myofibers can be seen in WT compared with Nrf2−/− mice (white arrow).
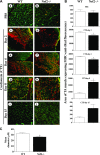
Figure 9.
Delayed expression of MHC-emb in TA muscle of Nrf2−/− mice injured with CTX. A) Cross sections of regenerating TA muscle of WT (a, c, e, g, i) and Nrf2−/− (b, d, f, h, j) mice showing anti-MHC-emb (red fluorescence). Muscle cell plasma membrane and nucleus were counter stained with anti-laminin (green) and DAPI (blue), respectively. Cross section of PBS-injected TA muscles of WT and Nrf2−/− mice (a, b), and cross-sections at 2 d (c, d), 4 d (e, f), 8 d (g, h), and 15 d (i, j) following CTX injury of TA muscle of WT and Nrf2−/− mice. B) Average area of TA muscle expressing MHC-emb of n = 3 section of each group was determined using ImageJ software. C) Average diameter of ∼100 green positive myotubes per field (Ai, j) of n = 3 sections of each group was determined using ImageJ software. n = 3/d per group. Statistical significance was calculated by 1-way ANOVA. *P < 0.05 CTX-WT vs. Nrf2−/−.
Figure 10.
CTX treatment hampers C2C12 myoblast differentiation and wound healing. A) Graph illustrates the percentage C2C12 cells viability measured by MTT assay in PBS and CTX treatment. B) Bright field light microscopy images showing the effect of 0.5 µM CTX treatment on C2C12 myoblast differentiation (×10 magnification). C) Artificial scratch wounds were created in Nrf2-silenced C2C12 monolayers, and the wound closure was captured by light microscope (×10 magnification). D) Graph represents the percentage of wound healing calculated by measuring the area of wound using ImageJ software. E) MTT cell viability assay of C2C12 myoblast transfected with control and Nrf2 shRNA for 48 h and treated with CTX (0.5 µM) for 24 h. Statistical significance was calculated by Student’s t test. *P < 0.05 between percent of wound healing of PBS and CTX-treated myoblast. ***P < 0.05 between control-shRNA+CTX vs. Nrf2-shRNA+CTX.
Figure 11.
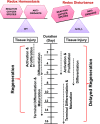
Schematic representation of the effect of Nrf2 deficiency on skeletal muscle regeneration after injury. Under normal physiologic conditions, when redox homeostasis is being maintained by the transcription factor Nrf2; activation, proliferation, and differentiation of satellite cells occurs to regenerate skeletal muscle after injury. However, when the redox imbalance occurs due to loss and/or inactivation of Nrf2, skeletal muscle regeneration could be delayed.
Similar articles
- Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells.
Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, Richardson RS, Gomes AV, Hoidal JR, Rajasekaran NS. Narasimhan M, et al. Free Radic Biol Med. 2014 Jun;71:402-414. doi: 10.1016/j.freeradbiomed.2014.02.023. Epub 2014 Mar 6. Free Radic Biol Med. 2014. PMID: 24613379 Free PMC article. - Autophagy controls neonatal myogenesis by regulating the GH-IGF1 system through a NFE2L2- and DDIT3-mediated mechanism.
Zecchini S, Giovarelli M, Perrotta C, Morisi F, Touvier T, Di Renzo I, Moscheni C, Bassi MT, Cervia D, Sandri M, Clementi E, De Palma C. Zecchini S, et al. Autophagy. 2019 Jan;15(1):58-77. doi: 10.1080/15548627.2018.1507439. Epub 2018 Sep 10. Autophagy. 2019. PMID: 30081710 Free PMC article. - Implication of basal lamina dependency in survival of Nrf2-null muscle stem cells via an antioxidative-independent mechanism.
Takemoto Y, Inaba S, Zhang L, Tsujikawa K, Uezumi A, Fukada SI. Takemoto Y, et al. J Cell Physiol. 2019 Feb;234(2):1689-1698. doi: 10.1002/jcp.27040. Epub 2018 Aug 2. J Cell Physiol. 2019. PMID: 30070693 - Nuclear Factor Erythroid 2-Related Factor 2 and Its Targets in Skeletal Muscle Repair and Regeneration.
Łoboda A, Dulak J. Łoboda A, et al. Antioxid Redox Signal. 2023 Mar;38(7-9):619-642. doi: 10.1089/ars.2022.0208. Epub 2023 Mar 2. Antioxid Redox Signal. 2023. PMID: 36597355 Review. - Unlocking peak performance: The role of Nrf2 in enhancing exercise outcomes and training adaptation in humans.
Martinez-Canton M, Galvan-Alvarez V, Martin-Rincon M, Calbet JAL, Gallego-Selles A. Martinez-Canton M, et al. Free Radic Biol Med. 2024 Nov 1;224:168-181. doi: 10.1016/j.freeradbiomed.2024.08.011. Epub 2024 Aug 14. Free Radic Biol Med. 2024. PMID: 39151836 Review.
Cited by
- Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models.
Wang Y, Lu J, Liu Y. Wang Y, et al. Int J Mol Sci. 2022 Nov 2;23(21):13380. doi: 10.3390/ijms232113380. Int J Mol Sci. 2022. PMID: 36362166 Free PMC article. Review. - The role of Nrf2 in acute and chronic muscle injury.
Bronisz-Budzyńska I, Kozakowska M, Podkalicka P, Kachamakova-Trojanowska N, Łoboda A, Dulak J. Bronisz-Budzyńska I, et al. Skelet Muscle. 2020 Dec 8;10(1):35. doi: 10.1186/s13395-020-00255-0. Skelet Muscle. 2020. PMID: 33287890 Free PMC article. - Nrf2 Deficiency Exacerbates the Decline in Swallowing and Respiratory Muscle Mass and Function in Mice with Aspiration Pneumonia.
Hashimoto H, Okazaki T, Honkura Y, Ren Y, Ngamsnae P, Hisaoka T, Koshiba Y, Suzuki J, Ebihara S, Katori Y. Hashimoto H, et al. Int J Mol Sci. 2024 Nov 4;25(21):11829. doi: 10.3390/ijms252111829. Int J Mol Sci. 2024. PMID: 39519380 Free PMC article. - ALDH3A1-mediated detoxification of reactive aldehydes contributes to distinct muscle responses to denervation and Amyotrophic Lateral Sclerosis progression.
Li A, Dong L, Li X, Yi J, Ma J, Zhou J. Li A, et al. bioRxiv [Preprint]. 2024 Dec 2:2024.12.02.626422. doi: 10.1101/2024.12.02.626422. bioRxiv. 2024. PMID: 39677625 Free PMC article. Preprint. - Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health.
Miller VJ, Villamena FA, Volek JS. Miller VJ, et al. J Nutr Metab. 2018 Feb 11;2018:5157645. doi: 10.1155/2018/5157645. eCollection 2018. J Nutr Metab. 2018. PMID: 29607218 Free PMC article. Review.
References
- Blaisdell F. W. (2002) The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc. Surg. 10, 620–630 - PubMed
- Ribchester R. R., Thomson D., Wood N. I., Hinks T., Gillingwater T. H., Wishart T. M., Court F. A., Morton A. J. (2004) Progressive abnormalities in skeletal muscle and neuromuscular junctions of transgenic mice expressing the Huntington’s disease mutation. Eur. J. Neurosci. 20, 3092–3114 - PubMed
- Chargé S. B., Rudnicki M. A. (2004) Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK079626/DK/NIDDK NIH HHS/United States
- R35 CA197222/CA/NCI NIH HHS/United States
- R03 AG042860/AG/NIA NIH HHS/United States
- R01 HL118067/HL/NHLBI NIH HHS/United States
- P30 AG050886/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases