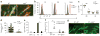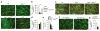Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β - PubMed (original) (raw)
doi: 10.1038/ni.3396. Epub 2016 Feb 22.
Lalit K Beura 2, Aleh Bobr 3, Brian Astry 1, Brian Chicoine 1, Sakeen W Kashem 1, Nathan E Welty 1, Botond Z Igyártó 1, Sathi Wijeyesinghe 1, Emily A Thompson 2, Catherine Matte 4 5, Laurent Bartholin 6, Alesia Kaplan 7, Dean Sheppard 8, Alina G Bridges 3, Warren D Shlomchik 9 10, David Masopust 2, Daniel H Kaplan 1 11 12
Affiliations
- PMID: 26901152
- PMCID: PMC5135085
- DOI: 10.1038/ni.3396
Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β
Javed Mohammed et al. Nat Immunol. 2016 Apr.
Abstract
Cells of the immune system that reside in barrier epithelia provide a first line of defense against pathogens. Langerhans cells (LCs) and CD8(+) tissue-resident memory T cells (TRM cells) require active transforming growth factor-β1 (TGF-β) for epidermal residence. Here we found that integrins αvβ6 and αvβ8 were expressed in non-overlapping patterns by keratinocytes (KCs) and maintained the epidermal residence of LCs and TRM cells by activating latent TGF-β. Similarly, the residence of dendritic cells and TRM cells in the small intestine epithelium also required αvβ6. Treatment of the skin with ultraviolet irradiation decreased integrin expression on KCs and reduced the availability of active TGF-β, which resulted in LC migration. Our data demonstrated that regulated activation of TGF-β by stromal cells was able to directly control epithelial residence of cells of the immune system through a novel mechanism of intercellular communication.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1
Activation of latent TGF-β by αvβ6 inhibits homeostatic LC migration. (a) Quantification of LCs in archival skin specimens from untreated (control) and losartan-treated patients, detected by immunohistochemistry with antibody to CD1a (anti-CD1a) or anti-langerin. Each symbol represents an individual patient; horizontal lines indicate the average. (b) Microscopy of whole mounts of back epidermis from cohorts (n = 10 mice in each) of wild-type mice (WT), TGF-βRI–CALC mice (with inducible expression of constitutively active TGF-βRI in LCs) and TGF-βLC mice (with inducible ablation of TGF-β in LCs) 9 d after the start of tamoxifen treatment, as well as epidermis from adult _Itgb6_−/− mice, all stained for MHC class II (green). (c) Ratio of the number of LCs in the epidermis (Epi) (obtained by counting of the MHCII+ cells in b) or skin draining LNs (LN) (obtained by flow cytometry) of _Itgb6_−/−, TGF-βRI–CALC and TGF-βLC mice to that in their wild-type counterparts. Each symbol represents an individual mouse (with results from the same mouse joined by a solid line); dashed horizontal lines indicate a ratio of 1.0. (d) Microscopy of whole mounts of ear epidermis from tamoxifen-treated wild-type and TGF-βRI–CALC mice given intradermal injection of anti-αvβ6 or isotype-matched control antibody (Isotype) (n = 4 mice per genotype per group), stained for MHC class II (green). (e) Quantification of LCs per high-power field (HPF) in the mice in d. Each symbol represents LCs per HPF; small horizontal lines indicate the average. Scale bars (b,d), 100 μm. NS, not significant (P > 0.05); *P < 0.01 and **P < 0.0001 (two-tailed unpaired Student’s t test (a,c) or Tukey’s multiple comparisons test (e)). Data are representative of experiments with n = 42 total donors (a) or are representative of (b,d) or pooled from (c,e) three independent experiments.
Figure 2
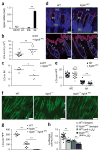
Epidermal residence of LCs requires expression of αvβ6 by IFE epidermal KCs. (a) Microscopy of whole mounts of back epidermis from wild-type and _Itgb6_−/− mice, stained for MHC class II (red) and αvβ6 (green); arrowheads indicate bulge region of telogen hair; asterisks indicate IFE region. (b) Flow cytometric analysis of the expression of αvβ6 by IM, IFE and bulge KCs (identified as in Supplementary Fig. 3b) from the back skin of wild-type and _Itgb6_−/− mice (key). (c) Quantification of LCs in transverse sections of back skin from wild-type and _Itgb6_−/− mice, identified by colocalization of langerin and MHC class II within the IFE region or IM. (d) Distance of IFE LCs from the hair follicle (HF) in sections as in c. (e) Quantitative RT-PCR analysis of Itgb6 mRNA in sorted epidermal populations of dendritic epidermal T cells (DETC), LCs, and IFE and IM KCs in wild-type mice; results are presented relative to those of the control gene Hprt. (f) Frequency of host- and donor-derived epidermal LCs in lethally irradiated wild-type→wild-type (WT→WT) or wild-type→_Itgb6_−/− (WT→_Itgb6_−/−) (CD45.2+) chimeras 6–8 weeks after transfer of BM from wild-type (CD45.1+) donors, analyzed by flow cytometry. (g) Microscopy of whole mounts of back epidermis from chimeras as in f, stained for MHC class II (green); autofluorescence shows hair shafts. Scale bars (a,g), 100 μm. ND, not detected. Each symbol (c,d,f) represents LCs per HPF (c), distance from HF (d) or an individual mouse (f); small horizontal lines indicate the mean. *P < 0.01 and **P < 0.0001 (two-tailed unpaired Student’s t test). Data are representative of three independent experiments with eight mice per genotype (a,b,g) or six independent experiments (e; mean + s.e.m.) or are pooled from two independent experiments with two mice per group (c,d) or three independent experiments (f).
Figure 3
LC residence is controlled by spatially distinct expression of αvβ6 and αvβ6 on KC subsets through activation of latent-TGF-β. (a) Quantitative RT-PCR analysis of Itgb8 mRNA in sorted epidermal populations from wild-type mice (as in Fig. 2e). (b) Quantification of LCs in skin-draining LNs of wild-type and _Itgb8_ΔKC mice. (c) Quantification of LCs (per HPF) in IM of wild-type and _Itgb8_ΔKC mice (as in Fig. 2c). (d) Microscopy of whole mounts of tail epidermis (top) and transverse sections of back skin (bottom) from wild-type and _Itgb8_ΔKC mice, stained for langerin (red) and with the DNA-binding dye DAPI (blue); dashed lines, dermal-epidermal junction; SG, sebaceous gland; asterisks (bottom), IM. (e) Quantification of LCs in the IFE region and IM of the stained transverse skin sections in d. (f) Microscopy of whole mounts of back epidermis from wild-type, _Itgb6_−/− and _Itgb6_−/−_Itgb8_ΔKC mice, stained for MHC class II (green); autofluorescence shows hair shafts. (g) Quantification of LCs in f. (h) Luciferase activity in mink lung TGF-β reporter cells cultured together with wild-type or _Itgb8_ΔKC primary KCs pre-incubated with anti-αvβ6 or isotype-matched control antibody; results are presented as relative light units (RLU), normalized to those of wild-type control cells. Each symbol (b,c,e,g,h) represents an individual mouse (b,c,g), LCs per HPF (e) or RLU values (f); small horizontal lines indicate the mean (±s.e.m. in h). Scale bars, 50 mu;m (d) or 100 mu;m (f). *P < 0.05 and **P < 0.0001 (two-tailed unpaired Student’s t test (a–c,e) or Tukey’s multiple-comparisons test (g,h)). Data are representative of six independent experiments (a; mean + s.e.m) or three independent experiments (d,f), or are pooled from three independent experiments (b,c,e,g) with n = 5 mice per group (d,e) or three to six independent experiments (h).
Figure 4
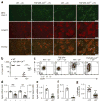
UV irradiation promotes LC migration through diminished integrin expression and TGF-β activation. (a) Microscopy of whole mounts of back epidermis from tamoxifen-treated wild-type and TGF-βRI–CALC mice (n = 5 per group) 4 d after sham treatment (-UV) or UVB irradiation (+UV), stained for MHC class II (green) and langerin (red). Scale bars, 100 mu;m. (b) Quantification of LCs (per HPF) in a. (c) Flow cytometry analyzing the expression of CD86 and CCR7 by epidermal LCs from mice as in a, gated as CD45+MHCII+CD11bint langerin-positive cells. Numbers in quadrants indicate percent cells in each. (d) Quantitative RT-PCR analysis of Itgb6 mRNA (left vertical axis) and Itgb8 mRNA (right vertical axis) in flow cytometry–sorted populations of IFE and IM KCs from wild-type mice 18 h after sham treatment or UVB irradiation (presented as in Fig. 2e). (e,f) Quantitative RT-PCR analysis of Itgb6 mRNA (e) and Itgb8 mRNA (f) in mouse primary KC cultures 24 h after sham treatment or UVB irradiation (presented as in Fig. 2e). (g) Luciferase activity of mink lung TGF-β reporter cells cultured together with primary keratinocytes given sham treatment or UVB irradiation; results are normalized to those of sham-treated cells. Each symbol (b,d–g) represents LCs per HPF (b), an independent experiment (d–f) or RLU values (g); small horizontal lines indicate the mean (±s.e.m. in d–g). *P < 0.01, **P < 0.001 and ***P < 0.0001 (two-tailed Mann-Whitney test (b,f) or two-tailed unpaired t test (d,e,g)). Data are representative of three independent experiments (a,c) or are pooled from three independent experiments with five mice per group (b) or six to seven independent experiments (d–g).
Figure 5
αvβ6 and αvβ8 are required for the residence of CD8+ TRM cells in epidermis. (a) Microscopy of whole mounts of back epidermis from wild-type, _Itgb6_−/−, _Itgb6_−/−_Itgb8_ΔKC and LC-deficient (ΔLC) mice given injection of Thy-1.1+ P14 cells on day -1 and infected with LCMV on day 0, followed by topical treatment with DNFB on day 3 (for epidermal seeding of P14 T cells), stained for Thy-1.1 (red) and MHC class II (green) on day 42 after infection. (b) Quantification of Thy-1.1+ cells in a. (c) Microscopy of whole mounts of back epidermis from wild-type, _Itgb6_−/− and _Itgb6_−/−_Itgb8_ΔKC treated as in a, stained for Thy-1.1 (red) and MHC class II (green) on day 7 after infection. (d) Quantification of Thy-1.1+ cells in c. (e) Frequency of CD103+Thy-1.1+ cells in epidermis of the mice in c, assessed by flow cytometry on day 7 after infection. (f) Microscopy of whole mounts of back epidermis from wild-type mice treated intraperitoneally with anti-αvβ6 or isotype-matched control antibody once a week for 4 weeks starting at 56 d after infection with LCMV and epidermal seeding of TRM cells as in a, stained for Thy-1.1 (red) and MHC class II (green). (g) Quantification of Thy-1.1+ cells in f. Autofluorescent hair follicles are visible in a,c,f. Scale bars (a,c,f), 100 μm. *P < 0.05, **P < 0.01 and ***P < 0.0001 (Tukey’s multiple-comparisons test (b,e) or two-tailed Mann-Whitney test (g)). Data are representative of two to three independent experiments with n = 4 mice per group (a,b; mean + s.e.m. in b) or two independent experiments with n = 4–5 mice per group (c–g; mean + s.e.m. in d,e,g).
Figure 6
αvβ6 is required for residence of TRM cells in intestinal epithelium. (a) Microscopy of small intestine from wild-type and _Itgb6_−/− mice 42 d after infection with LCMV, stained for Thy-1.1 (red) and collagen IV (ColIV) (green) and with DAPI (blue); arrowheads indicate TRM cells in the epithelial layer. (b,c) Quantification of Thy-1.1+ cells (per 106 nucleated cells) in the epithelial layer (above collagen IV (arrowheads) in a) (b) and lamina propria (LP) (below collagen IV in a) (c) of the small intestine (SI) of mice as in a. (d) Microscopy of small intestine from wild-type and _Itgb6_−/− mice 7 d after infection with LCMV, stained as in a. (e,f) Quantification of Thy-1.1+ cells in the epithelial layer (as in b) (e) and lamina propria (as in c) (f) of the small intestine of mice as in d. (g) Microscopy of small intestine from wild-type mice infected with LCMV and then treated intraperitoneally with anti-αvβ6 or isotype-matched control antibody starting at 56 d after infection, stained as in a. (h,i) Quantification of Thy-1.1+ cells in the epithelial layer (as in b) (h) and lamina propria (as in c) (i) of the small intestine of mice as in g. Scale bars (a,d,g), 50 μm. *P < 0.05 (two-tailed Mann-Whitney test). Data representative of three independent experiments with n = 4 mice per group (a–c; mean + s.e.m. in b,c) or two independent experiments with n = 4–5 mice per group (d–i; mean + s.e.m. in e,f,h,i).
Similar articles
- Circulating memory CD8+ T cells are limited in forming CD103+ tissue-resident memory T cells at mucosal sites after reinfection.
Behr FM, Beumer-Chuwonpad A, Kragten NAM, Wesselink TH, Stark R, van Gisbergen KPJM. Behr FM, et al. Eur J Immunol. 2021 Jan;51(1):151-166. doi: 10.1002/eji.202048737. Epub 2020 Aug 31. Eur J Immunol. 2021. PMID: 32762051 - Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma.
Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, Saya H, Amagai M, Nagao K. Adachi T, et al. Nat Med. 2015 Nov;21(11):1272-9. doi: 10.1038/nm.3962. Epub 2015 Oct 19. Nat Med. 2015. PMID: 26479922 Free PMC article. - Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis.
Kim BS, Miyagawa F, Cho YH, Bennett CL, Clausen BE, Katz SI. Kim BS, et al. J Invest Dermatol. 2009 Dec;129(12):2805-17. doi: 10.1038/jid.2009.176. Epub 2009 Jun 25. J Invest Dermatol. 2009. PMID: 19554018 Free PMC article. - Cytokine-mediated communication by keratinocytes and Langerhans cells with dendritic epidermal T cells.
Takashima A, Bergstresser PR. Takashima A, et al. Semin Immunol. 1996 Dec;8(6):333-9. doi: 10.1006/smim.1996.0044. Semin Immunol. 1996. PMID: 8961384 Review. - TGF-β: Many Paths to CD103+ CD8 T Cell Residency.
Qiu Z, Chu TH, Sheridan BS. Qiu Z, et al. Cells. 2021 Apr 23;10(5):989. doi: 10.3390/cells10050989. Cells. 2021. PMID: 33922441 Free PMC article. Review.
Cited by
- Resident memory T cells form during persistent antigen exposure leading to allograft rejection.
Abou-Daya KI, Tieu R, Zhao D, Rammal R, Sacirbegovic F, Williams AL, Shlomchik WD, Oberbarnscheidt MH, Lakkis FG. Abou-Daya KI, et al. Sci Immunol. 2021 Mar 19;6(57):eabc8122. doi: 10.1126/sciimmunol.abc8122. Sci Immunol. 2021. PMID: 33741656 Free PMC article. - Decoding Tissue-Residency: Programming and Potential of Frontline Memory T Cells.
Park SL, Mackay LK. Park SL, et al. Cold Spring Harb Perspect Biol. 2021 Oct 1;13(10):a037960. doi: 10.1101/cshperspect.a037960. Cold Spring Harb Perspect Biol. 2021. PMID: 33753406 Free PMC article. Review. - Epithelial organoid supports resident memory CD8 T cell differentiation.
Ulibarri MR, Lin Y, Ramprashad JC, Han G, Hasan MH, Mithila FJ, Ma C, Gopinath S, Zhang N, Milner JJ, Beura LK. Ulibarri MR, et al. Cell Rep. 2024 Aug 27;43(8):114621. doi: 10.1016/j.celrep.2024.114621. Epub 2024 Aug 15. Cell Rep. 2024. PMID: 39153200 Free PMC article. - Type I Interferons Enhance the Repair of Ultraviolet Radiation-Induced DNA Damage and Regulate Cutaneous Immune Suppression.
Sherwani MA, Ahmad I, Lewis MJ, Abdelgawad A, Rashid H, Yang K, Chen CY, Raman C, Elmets CA, Yusuf N. Sherwani MA, et al. Int J Mol Sci. 2022 Feb 5;23(3):1822. doi: 10.3390/ijms23031822. Int J Mol Sci. 2022. PMID: 35163747 Free PMC article. - Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis.
Otsuka M, Egawa G, Kabashima K. Otsuka M, et al. Front Immunol. 2018 Jul 30;9:1768. doi: 10.3389/fimmu.2018.01768. eCollection 2018. Front Immunol. 2018. PMID: 30105033 Free PMC article. Review.
References
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR060744/AR/NIAMS NIH HHS/United States
- R37 AI084913/AI/NIAID NIH HHS/United States
- R01 AI084913/AI/NIAID NIH HHS/United States
- R01 AR060744/AR/NIAMS NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- F30 DK097856/DK/NIDDK NIH HHS/United States
- AI084913/AI/NIAID NIH HHS/United States
- T32 AI007313/AI/NIAID NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous