A Family of Salmonella Type III Secretion Effector Proteins Selectively Targets the NF-κB Signaling Pathway to Preserve Host Homeostasis - PubMed (original) (raw)
A Family of Salmonella Type III Secretion Effector Proteins Selectively Targets the NF-κB Signaling Pathway to Preserve Host Homeostasis
Hui Sun et al. PLoS Pathog. 2016.
Abstract
Microbial infections usually lead to host innate immune responses and inflammation. These responses most often limit pathogen replication although they can also result in host-tissue damage. The enteropathogenic bacteria Salmonella Typhimurium utilizes a type III secretion system to induce intestinal inflammation by delivering specific effector proteins that stimulate signal transduction pathways resulting in the production of pro-inflammatory cytokines. We show here that a family of related Salmonella Typhimurium effector proteins PipA, GogA and GtgA redundantly target components of the NF-κB signaling pathway to inhibit transcriptional responses leading to inflammation. We show that these effector proteins are proteases that cleave both the RelA (p65) and RelB transcription factors but do not target p100 (NF-κB2) or p105 (NF-κB1). A Salmonella Typhimurium strain lacking these effectors showed increased ability to stimulate NF-κB and increased virulence in an animal model of infection. These results indicate that bacterial pathogens can evolve determinants to preserve host homeostasis and that those determinants can reduce the pathogen's virulence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Fig 1. Absence of the PipA-family of effector proteins increases the mouse virulence of S. Typhimurium without increasing bacterial loads.
(A and B) C57/BL6 nramp +/+ mice were orally (A) or intraperitoneally (B) infected with wild-type S. Typhimurium or the ΔpipA ΔgogA ΔgtgA mutant and bacterial loads in the indicated tissues enumerated 7 days after infection. Each circle represents the bacterial load for an individual animal and horizontal bars indicate geometric means. The results are the combination of two independent experiments. (C and D) Survival of animals orally (C) or intraperitoneally (D) infected with wild-type S. Typhimurium (n = 7) or the ΔpipA ΔgogA ΔgtgA mutant (n = 8) strains 6 days after infection. The p values of the difference in the survival of animals infected with wild type or mutant strains determined by the log-rank test are shown. The results are the combination of two independent experiments.
Fig 2. Absence of the PipA-family of effector proteins increases the ability of S. Typhimurium to stimulate pro-inflammatory cytokine expression in the mouse intestine.
(A and B) C57/BL6 nramp +/+ mice were orally infected with wild type (n = 5) or ΔpipA ΔgogA ΔgtgA (n = 5) S. Typhimurium strains and 24 (A) or 48 (B) hours after infection the relative levels of the indicated cytokines in the intestine were measured by quantitative PCR. Data were normalized to the levels of GAPDH and are expressed relative to uninfected control animals (n = 5). The data shown were compiled from two independent experiments of three measurements each. p values of the indicated differences determined by the Student t test are shown.
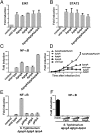
Fig 3. The PipA family of effector proteins negatively regulates _Salmonella_-induced NF-κB signaling.
(A-E) HEK293T cells were transfected with plasmids encoding Elk1 (A), STAT3 (B) or NF-κB (C-E) signaling reporter constructs. Eighteen hours after transfection, cells were infected with the indicated S. Typhimurium strains at a MOI = 5 and the reporter activity was measured at 8 hours after infection (A-C, E) or at the indicated times after infection (D). Data are shown relative to the activity of the reporter in uninfected control cells and represent the mean ± standard deviation of three independent measurements. (F) HEK293T cells were transfected with a plasmid encoding a NF-κB reporter construct along with 25 or 50 ng of a plasmid encoding PipA, GtgA, or GogA. Eighteen hours after transfection, cells were infected with S. Typhimurium Δ_gog_A Δ_gtg_A Δ_pip_A at a MOI = 5 and the reporter activity was measured 8 hs after infection. Data are shown relative to the activity of the reporter in uninfected control cells and represent the mean ± standard deviation of three independent measurements.
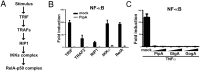
Fig 4. The PipA family of effector proteins can directly inhibit NF-κB signaling.
(A) Simplified diagram of the TRIF signaling pathway. (B) HEK293T cells were transfected with a plasmid encoding a NF-κB reporter construct along with plasmids encoding the indicated proteins (horizontal axis) in conjunction with a plasmid encoding PipA or the empty vector (mock). The reporter activity was subsequently measured 18 hrs after transfection. Data are shown relative to the activity of the reporter in control cells (transfected with the empty vector) and represent the mean ± standard deviation of three independent measurements. (C) HEK293T cells were transfected with a plasmid encoding a NF-κB reporter construct along with 25 or 50 ng of a plasmid encoding PipA, GtgA or GogA. Eighteen hours after transfection, cells were treated with TNFα (10 ng/ml) or infected with the Δ_pip_A Δ_gog_A Δ_gtg_A S. Typhimurium at a MOI = 5 and the reporter activity was measured 8 hs after treatment. Data are shown relative to the activity of the reporter in uninfected control cells and represent the mean ± standard deviation of three independent measurements.
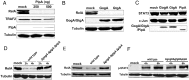
Fig 5. PipA, GtgA and GogA inhibit the NF-κB pathway by targeting RelA.
(A) HEK293T cells were transiently transfected with plasmids encoding M45-tagged RelA or HA-tagged TRAF2, along with the indicated amounts of a plasmid encoding FLAG-tagged PipA or the empty vector (mock). Eighteen hours after transfection, cell lysates were analyzed by Western blot with anti-M45, anti-HA, anti-FLAG, and anti tubulin antibodies (as loading control). (B) HEK293T cells were transiently transfected with plasmids encoding M45-tagged RelA, along with plasmids encoding FLAG-tagged GogA, or GtgA or the empty vector (control). Eighteen hours after transfection, cell lysates were analyzed by Western blot with anti-M45 and anti-FLAG antibodies. (C) HEK293T cells were transiently transfected with plasmids encoding M45-tagged STAT3, or c-Jun along with plasmids encoding FLAG-tagged PipA, GogA, or GtgA or the empty vector (mock). Eighteen hours after transfection, cell lysates were analyzed by Western blot with anti-M45 and anti-FLAG antibodies. (D) HEK293T cells were transiently transfected with a plasmid encoding M45-tagged RelA. Eighteen hours after transfection, cells were infected with wild type or the ΔpipA ΔgogA ΔgtgA S. Typhimurium strains with a MOI = 10. Cell lysates were analyzed by western blot with anti-M45 or anti tubulin (as loading control) antibodies at the indicated times after infection. (E) HeLa cells were infected with wild type or the Δ_pip_A Δ_gog_A Δ_gtg_A S. Typhimurium strains with a MOI = 10. 6 hs after infection, cell lysates were analyzed by Western blot with anti-RelA, and anti-tubulin antibodies (as loading control). (F) HeLa cells were infected with wild type or the Δ_pip_A Δ_gog_A Δ_gtg_A S. Typhimurium strains with a MOI = 10. At indicated times after infection, cell lysates were analyzed by Western blot with anti phosphorylated STAT3 and anti-tubulin antibodies (as loading control).
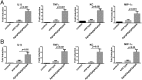
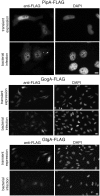
Fig 6. The PipA family of effector proteins localizes to the nucleus of infected cells.
Immunofluorescence staining of HeLa cells transfected with a plasmid encoding FLAG-tagged PipA, GogA, or GtgA or infected with S. Typhimurium encoding FLAG-tagged PipA, GogA, or GtgA. Eighteen hours after transfection and 4 hours after bacterial infection, cells were stained with an anti FLAG antibody (to visualize the effector proteins) and 4',6-diamidino-2-phenylindole (DAPI) to visualize nuclear DNA. Scale bars: 5 and 10 μm for PipA and GogA/GtgA, respectively.
Fig 7. PipA, GtgA and GogA are specific proteases for transcription factors of the RelA family.
(A) HEK293T cells were transiently transfected with plasmids encoding M45-tagged RelA, along with plasmids encoding either FLAG-tagged PipA or its catalytic mutant PipAE181A. Eighteen hours after transfection, cell lysates were analyzed by Western blot with anti-M45, anti-FLAG, and anti-tubulin (as loading control) antibodies. (B) HEK293T cells were transiently transfected with a plasmid encoding a NF-κB reporter construct, along with 25, 50 and 100 ng of plasmid DNA encoding either FLAG-tagged PipA or its catalytic mutant PipAE181A. Eighteen hours after transfection, cells were infected with the Δ_gog_A/Δ_gtg_A/Δ_pip_A S. Typhimurium mutant strain at MOI = 5 and the reporter activity measured 8 hs after infection. Data are the reporter activity relative to control cells (no infection) and represent the mean ± standard deviation of three independent experiments. (C) Purified RelA1-210 (8μg) was incubated with purified PipA or PipAE181A (2.5 μg) in a reaction buffer (40 μl) in the presence [50 (+) or 100 (++) mM] or absence (-) of EDTA. The reaction was stopped by addition of SDS loading buffer, proteins separated by SDS—PAGE and visualized by Coomassie blue staining. RelA1-210 and its cleaved product are indicated. (D) Purified RelA1-210 was incubated with purified GogA, GtgA, or PipA in a reaction buffer. The reaction was stopped by addition of SDS loading buffer, proteins separated by SDS—PAGE and visualized by Coomassie blue staining. RelA1-210 and its cleaved product are indicated (note that differences in cleavage efficiency between panels C and D are likely the result of differences in the solubility of the RelA preparation, which tended to aggregate after even limited storage). (E) HEK293T cells were transiently transfected with plasmids encoding M45-tagged RelB, p105, or p100, along with plasmids encoding FLAG-tagged PipA, GogA, or GtgA or the empty vector (mock). Eighteen hours after transfection, cell lysates were analyzed by western blot with anti-M45 and anti-FLAG antibodies.
Similar articles
- A Salmonella type III effector, PipA, works in a different manner than the PipA family effectors GogA and GtgA.
Takemura M, Haneda T, Idei H, Miki T, Okada N. Takemura M, et al. PLoS One. 2021 Mar 18;16(3):e0248975. doi: 10.1371/journal.pone.0248975. eCollection 2021. PLoS One. 2021. PMID: 33735297 Free PMC article. - Structure-function analyses of the bacterial zinc metalloprotease effector protein GtgA uncover key residues required for deactivating NF-κB.
Jennings E, Esposito D, Rittinger K, Thurston TLM. Jennings E, et al. J Biol Chem. 2018 Sep 28;293(39):15316-15329. doi: 10.1074/jbc.RA118.004255. Epub 2018 Jul 26. J Biol Chem. 2018. PMID: 30049795 Free PMC article. - Salmonella stimulates pro-inflammatory signalling through p21-activated kinases bypassing innate immune receptors.
Sun H, Kamanova J, Lara-Tejero M, Galán JE. Sun H, et al. Nat Microbiol. 2018 Oct;3(10):1122-1130. doi: 10.1038/s41564-018-0246-z. Epub 2018 Sep 17. Nat Microbiol. 2018. PMID: 30224799 Free PMC article. - Interactions between Salmonella and host macrophages - Dissecting NF-κB signaling pathway responses.
Yang F, Sheng X, Huang X, Zhang Y. Yang F, et al. Microb Pathog. 2021 May;154:104846. doi: 10.1016/j.micpath.2021.104846. Epub 2021 Mar 9. Microb Pathog. 2021. PMID: 33711426 Review. - Host Cell Targeting by Enteropathogenic Bacteria T3SS Effectors.
Pinaud L, Sansonetti PJ, Phalipon A. Pinaud L, et al. Trends Microbiol. 2018 Apr;26(4):266-283. doi: 10.1016/j.tim.2018.01.010. Epub 2018 Feb 21. Trends Microbiol. 2018. PMID: 29477730 Review.
Cited by
- Speaking the host language: how Salmonella effector proteins manipulate the host.
Pillay TD, Hettiarachchi SU, Gan J, Diaz-Del-Olmo I, Yu XJ, Muench JH, Thurston TLM, Pearson JS. Pillay TD, et al. Microbiology (Reading). 2023 Jun;169(6):001342. doi: 10.1099/mic.0.001342. Microbiology (Reading). 2023. PMID: 37279149 Free PMC article. Review. - Identification of Salmonella Pullorum Factors Affecting Immune Reaction in Macrophages from the Avian Host.
Fei X, Li Q, Jiao X, Olsen JE. Fei X, et al. Microbiol Spectr. 2023 Jun 15;11(3):e0078623. doi: 10.1128/spectrum.00786-23. Epub 2023 May 16. Microbiol Spectr. 2023. PMID: 37191575 Free PMC article. - A Salmonella type III effector, PipA, works in a different manner than the PipA family effectors GogA and GtgA.
Takemura M, Haneda T, Idei H, Miki T, Okada N. Takemura M, et al. PLoS One. 2021 Mar 18;16(3):e0248975. doi: 10.1371/journal.pone.0248975. eCollection 2021. PLoS One. 2021. PMID: 33735297 Free PMC article. - Evaluating the effect of spaceflight on the host-pathogen interaction between human intestinal epithelial cells and Salmonella Typhimurium.
Barrila J, Sarker SF, Hansmeier N, Yang S, Buss K, Briones N, Park J, Davis RR, Forsyth RJ, Ott CM, Sato K, Kosnik C, Yang A, Shimoda C, Rayl N, Ly D, Landenberger A, Wilson SD, Yamazaki N, Steel J, Montano C, Halden RU, Cannon T, Castro-Wallace SL, Nickerson CA. Barrila J, et al. NPJ Microgravity. 2021 Mar 9;7(1):9. doi: 10.1038/s41526-021-00136-w. NPJ Microgravity. 2021. PMID: 33750813 Free PMC article. - Phylogenetic analysis revealed that Salmonella Typhimurium ST313 isolated from humans and food in Brazil presented a high genomic similarity.
Seribelli AA, Gonzales JC, de Almeida F, Benevides L, Cazentini Medeiros MI, Dos Prazeres Rodrigues D, de C Soares S, Allard MW, Falcão JP. Seribelli AA, et al. Braz J Microbiol. 2020 Mar;51(1):53-64. doi: 10.1007/s42770-019-00155-6. Epub 2019 Nov 15. Braz J Microbiol. 2020. PMID: 31728978 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases