The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective - PubMed (original) (raw)
Review
The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective
Tomoki Kimura et al. Int J Mol Sci. 2016.
Abstract
Around 3000 proteins are thought to bind zinc in vivo, which corresponds to ~10% of the human proteome. Zinc plays a pivotal role as a structural, catalytic, and signaling component that functions in numerous physiological processes. It is more widely used as a structural element in proteins than any other transition metal ion, is a catalytic component of many enzymes, and acts as a cellular signaling mediator. Thus, it is expected that zinc metabolism and homeostasis have sophisticated regulation, and elucidating the underlying molecular basis of this is essential to understanding zinc functions in cellular physiology and pathogenesis. In recent decades, an increasing amount of evidence has uncovered critical roles of a number of proteins in zinc metabolism and homeostasis through influxing, chelating, sequestrating, coordinating, releasing, and effluxing zinc. Metallothioneins (MT) and Zrt- and Irt-like proteins (ZIP) and Zn transporters (ZnT) are the proteins primarily involved in these processes, and their malfunction has been implicated in a number of inherited diseases such as acrodermatitis enteropathica. The present review updates our current understanding of the biological functions of MTs and ZIP and ZnT transporters from several new perspectives.
Keywords: ZIP and ZnT transporter; chaperone; metallothionein; zinc.
Figures
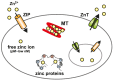
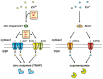
Figure 1
Cellular zinc homeostasis is controlled by the cooperative function of metallothioneins (MT) and Zrt- and Irt-like proteins (ZIP) and Zn transporters (ZnT). The mobilization of zinc into or out of the cytosol is directed by two zinc transporter families, ZIP and ZnT. In the cytosol, MTs bind zinc to reserve, buffer, and chelate. Zinc is compartmentalized into or out of intracellular organelles and vesicles by ZnT and ZIP transporters. Because of the binding of zinc to many different proteins, the free zinc ion concentration in the cytosol is estimated to be well below pM–low nM levels.
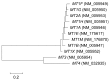
Figure 2
Phylogenetic tree of MT genes. The tree was constructed using coding sequences from NCBI RefSeq and the neighbor-joining method using MEGA6 software.
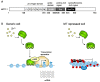
Figure 3
Expression regulation of MT gene expression. (A) Schematic representation of human metal response element-binding transcription factor-1 (MTF-1). The regions of the six-zinc fingers (F1–F6), acidic, proline-rich, and serine/threonine-rich domains are indicated by boxes and amino acid numbers; (B) Proposed molecular mechanisms in MT transcription in response to increases of intracellular free zinc. In generic cells, MTF-1 recruits the histone acetyltransferase p300 and increases MT transcription. In MT-repressed cells such as lymphosarcoma cells, and cancer cells, the promoter is highly methylated. DNA methyltransferase (DNMT) and methyl CpG binding proteins (MBD) are involved in the suppression. The epigenetic mechanism is described in Section 4.2. Ac, acetyl group; Me, methyl group; blue circle with two lines, nucleosome.
Figure 4
The subcellular localization of ZIP and ZnT transporters. The primary localization of ZIP (red arrows) and ZnT (green arrows) transporters is shown according to available information. This schematic illustrates a static view of their localization. Cytosolic zinc is mobilized into or out of different subcellular compartments, including synaptic vesicles or insulin granules in a cell-specific manner. ER, endoplasmic reticulum; TGN, _trans_-Golgi network.
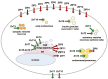
Figure 5
Cooperative function of MT, ZnT1, and ZnT4 in the activation of zinc-requiring ectoenzymes. The facilitated transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers may function under cooperative control of ZnT1, MT, and ZnT4 (left). ZnT1MTZnT4 KO cells exhibit significantly reduced TNAP activity (left), which is reminiscent of the phenotypes of cytosolic copper chaperone _Atox1_-deficient cells (right). Atox1 plays a crucial role as a copper chaperone in transferring cytosolic copper to two copper-transporting P-type ATPases, ATP7A and ATP7B, located in the _trans_-Golgi network (TGN). This therefore contributes to the activation of copper-requiring ectoenzymes (cuproenzymes). Considering the high level of analogy between ZnT1MTZnT4 KO and _Atox1_-deficient cells, a putative zinc chaperone under the cooperative control of ZnT1, MT, and ZnT4 is hypothesized to play a crucial role in facilitating the transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers (not shown) located in the early secretory pathway (ESP). This then contributes to the proper activation of zinc-requiring ectoenzymes such as TNAP (left).
Similar articles
- The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism.
Kambe T, Tsuji T, Hashimoto A, Itsumura N. Kambe T, et al. Physiol Rev. 2015 Jul;95(3):749-84. doi: 10.1152/physrev.00035.2014. Physiol Rev. 2015. PMID: 26084690 Review. - Current understanding of ZIP and ZnT zinc transporters in human health and diseases.
Kambe T, Hashimoto A, Fujimoto S. Kambe T, et al. Cell Mol Life Sci. 2014 Sep;71(17):3281-95. doi: 10.1007/s00018-014-1617-0. Epub 2014 Apr 8. Cell Mol Life Sci. 2014. PMID: 24710731 Free PMC article. Review. - Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica.
Hoch E, Levy M, Hershfinkel M, Sekler I. Hoch E, et al. Int J Mol Sci. 2020 Jan 22;21(3):734. doi: 10.3390/ijms21030734. Int J Mol Sci. 2020. PMID: 31979155 Free PMC article. - Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells.
Bin BH, Seo J, Kim ST. Bin BH, et al. J Immunol Res. 2018 Oct 2;2018:9365747. doi: 10.1155/2018/9365747. eCollection 2018. J Immunol Res. 2018. PMID: 30370308 Free PMC article. Review. - Zinc Homeostasis in Bone: Zinc Transporters and Bone Diseases.
Huang T, Yan G, Guan M. Huang T, et al. Int J Mol Sci. 2020 Feb 12;21(4):1236. doi: 10.3390/ijms21041236. Int J Mol Sci. 2020. PMID: 32059605 Free PMC article. Review.
Cited by
- Biochemical Markers of Zinc Nutrition.
Wang X, Zhang M, Ma J, Tie Y, Wang S. Wang X, et al. Biol Trace Elem Res. 2024 Dec;202(12):5328-5338. doi: 10.1007/s12011-024-04091-x. Epub 2024 Feb 6. Biol Trace Elem Res. 2024. PMID: 38319550 Review. - Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review.
Acevedo S, Segovia MF, de la Fuente-Ortega E. Acevedo S, et al. Nutrients. 2024 Jun 26;16(13):2026. doi: 10.3390/nu16132026. Nutrients. 2024. PMID: 38999774 Free PMC article. Review. - Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis.
Pompano LM, Boy E. Pompano LM, et al. Adv Nutr. 2021 Feb 1;12(1):141-160. doi: 10.1093/advances/nmaa087. Adv Nutr. 2021. PMID: 32722790 Free PMC article. - Non-Cell-Autonomous Regulation of Optic Nerve Regeneration by Amacrine Cells.
Sergeeva EG, Rosenberg PA, Benowitz LI. Sergeeva EG, et al. Front Cell Neurosci. 2021 Apr 16;15:666798. doi: 10.3389/fncel.2021.666798. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33935656 Free PMC article. Review. - Synaptic Zinc: An Emerging Player in Parkinson's Disease.
Sikora J, Ouagazzal AM. Sikora J, et al. Int J Mol Sci. 2021 Apr 29;22(9):4724. doi: 10.3390/ijms22094724. Int J Mol Sci. 2021. PMID: 33946908 Free PMC article. Review.
References
- Thiers R.E., Vallee B.L. Distribution of metals in subcellular fractions of rat liver. J. Biol. Chem. 1957;226:911–920. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources