YAP/TAZ Are Mechanoregulators of TGF- β-Smad Signaling and Renal Fibrogenesis - PubMed (original) (raw)
. 2016 Oct;27(10):3117-3128.
doi: 10.1681/ASN.2015050499. Epub 2016 Mar 9.
Masahiro Narimatsu 3, Mingliang Lu 1, Xiaolin He 1, Ahmad M Sidiqi 1 2, Monica F Tolosa 1 4, Lauren Chan 1, Krystale De Freitas 1, Janne Folke Bialik 1, Syamantak Majumder 1, Stellar Boo 5, Boris Hinz 5, Qinghong Dan 1, Andrew Advani 1 2, Rohan John 6, Jeffrey L Wrana 3, Andras Kapus 1 2, Darren A Yuen 7 2 4
Affiliations
- PMID: 26961347
- PMCID: PMC5042658
- DOI: 10.1681/ASN.2015050499
YAP/TAZ Are Mechanoregulators of TGF- _β_-Smad Signaling and Renal Fibrogenesis
Stephen G Szeto et al. J Am Soc Nephrol. 2016 Oct.
Abstract
Like many organs, the kidney stiffens after injury, a process that is increasingly recognized as an important driver of fibrogenesis. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are related mechanosensory proteins that bind to Smad transcription factors, the canonical mediators of profibrotic TGF-β responses. Here, we investigated the role of YAP/TAZ in the matrix stiffness dependence of fibroblast responses to TGF-β In contrast to growth on a stiff surface, fibroblast growth on a soft matrix led to YAP/TAZ sequestration in the cytosol and impaired TGF-_β_-induced Smad2/3 nuclear accumulation and transcriptional activity. YAP knockdown or treatment with verteporfin, a drug that was recently identified as a potent YAP inhibitor, elicited similar changes. Furthermore, verteporfin reduced YAP/TAZ levels and decreased the total cellular levels of Smad2/3 after TGF-β stimulation. Verteporfin treatment of mice subjected to unilateral ureteral obstruction similarly reduced YAP/TAZ levels and nuclear Smad accumulation in the kidney, and attenuated renal fibrosis. Our data suggest that organ stiffening cooperates with TGF-β to induce fibrosis in a YAP/TAZ- and Smad2/3-dependent manner. Interference with this YAP/TAZ and TGF-β/Smad crosstalk likely underlies the antifibrotic activity of verteporfin. Finally, through repurposing of a clinically used drug, we illustrate the therapeutic potential of a novel mechanointerference strategy that blocks TGF-β signaling and renal fibrogenesis.
Keywords: TAZ; TGF-beta; YAP; chronic kidney disease; extracellular matrix; fibrosis.
Copyright © 2016 by the American Society of Nephrology.
Figures
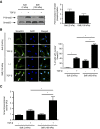
Figure 1.
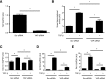
Substrate stiffness modulates TGF-_β_–induced Smad signaling via regulation of intracellular localization of Smad2 and Smad3. (A and B) NRK49F rat renal fibroblasts cultured on soft (2 kPa) and stiff (100 kPa) fibronectin–coated gels were stimulated with TGF-β followed by analysis of (A) Smad2 phosphorylation and (B) intracellular Smad2/3 localization. In A, quantification of the TGF-_β_–induced fold increase in Smad2 phosphorylation is presented (_n_=4 per condition). In B, cells were counterstained with DAPI to identify nuclei (_n_=3 coverslips per condition). Arrows point to cells with predominantly nuclear Smad2/3 staining. Scale bar, 20 _μ_m. (C) Fibroblasts transfected with a Smad–binding element luciferase reporter and a normalizing Renilla luciferase control were stimulated with TGF-β, and the resultant luminescence was measured (_n_=6 per condition). One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; P-Smad2, phospho-Smad2. *P<0.05.
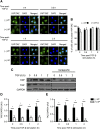
Figure 2.
Verteporfin treatment results in dramatically reduced levels of YAP and TAZ. NRK49F fibroblasts were treated with or without 250 nmol/L verteporfin for a total of 12 hours. TGF-β (10 ng/ml) was added for the indicated times. In A, after treatment with verteporfin and TGF-β as described above, NRK49F fibroblasts were stained with an antibody directed against YAP/TAZ (green) and counterstained with DAPI (blue) to identify nuclei. Representative images are shown showing loss of whole–cell YAP/TAZ staining even before TGF-β stimulation but no change in the predominantly nuclear localization of YAP/TAZ. Scale bar, 15 _μ_m. (B) The percentage of cells with predominantly nuclear YAP/TAZ staining was quantified at each time point. A minimum of 50 cells was counted per replicate (four replicates per condition). In C, after treatment with verteporfin and TGF-β, lysates were immunoblotted for YAP, TAZ, and GAPDH. Representative blots are shown in C, and quantification of relative levels of (D) YAP (_n_=3 per condition) and (E) TAZ (_n_=5 per condition) are also shown. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. *P<0.05.
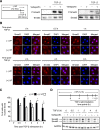
Figure 3.
Verteporfin treatment impairs TGF-_β_–induced Smad2/3 signaling via reducing the nuclear accumulation and total levels of Smad2/3 protein. (A) NRK49F renal fibroblasts grown in fibronectin–coated plastic dishes were treated with and without 250 nmol/L verteporfin for 12 hours and stimulated with or without TGF-β for 30 minutes. Lysates were immunoblotted for phospho-Smad2, phospho-Smad3, Smad2, or Smad3. Blots shown are representative of three replicates. (B) NRK49F fibroblasts grown on fibronectin-coated coverslips were treated with or without 250 nmol/L verteporfin for a total of 12 hours and 10 ng/ml TGF-β for the indicated times. Cells were stained with an anti-Smad2 antibody (red) and counterstained with DAPI (blue) to identify nuclei. Representative images demonstrate not only reduced nuclear accumulation of Smad2 beginning early post–TGF-β (0.5–1 hours) but also, diminished whole–cell Smad2 levels at later time points (2, 4, and 8 hours). Scale bar, 15 _μ_m. (C) The percentage of cells with predominantly nuclear Smad2 staining was quantified at each time point. A minimum of 50 cells was counted per replicate (four replicates per condition). One-way ANOVA with post hoc Fisher least significant difference was performed. *P<0.05. In D, NRK49F fibroblasts grown on fibronectin-coated plastic were treated with 250 nmol/L verteporfin for a total of 12 hours and 10 ng/ml TGF-β for the indicated times. Verteporfin treatment resulted in a dramatic reduction in Smad2 and Smad3 post–TGF-β, a setting in which Smad2 and Smad3 are primarily in their activated state. P-Smad2, phospho-Smad2; P-Smad3, phospho-Smad3; VP, verteporfin.
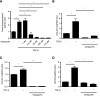
Figure 4.
Verteporfin treatment attenuates TGF-β/Smad–induced profibrotic gene expression. (A) NRK49F fibroblasts grown in fibronectin–coated plastic dishes were transfected with a Firefly luciferase reporter driven by four tandem Smad–binding elements and then treated with or without 250 nmol/L verteporfin for 4 hours followed by the addition of 10 ng/ml TGF-β for 20 hours. Twenty-four hours post-transfection, Smad3-dependent transcription was assessed using a Smad–dependent luciferase reporter activity assay (_n_=3 replicates per condition). (B–D) NRK49F cells were treated with or without 250 nmol/L verteporfin for 2 hours followed by stimulation with or without TGF-β for 24 hours. mRNA levels of (B) COL3A1, (C) COL4A1, and (D) ACTA2, normalized to GAPDH, were measured by quantitative RT-PCR. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit. *P<0.05.
Figure 5.
YAP silencing inhibits the TGF-β/Smad–induced increase in profibrotic gene expression. NRK49F renal fibroblasts were transfected with siRNA directed against YAP or a scrambled siRNA. (A) Forty-eight hours post-transfection, knockdown of YAP was confirmed via quantitative RT-PCR. The effects of YAP knockdown on TGF-_β_–induced Smad–dependent transcription were assessed (B) using a Smad–dependent luciferase reporter activity assay and via measurement of endogenous mRNA levels of the TGF-β/Smad–inducible genes (C) COL1A1, (D) ACTA2, and (E) PAI1. Results are representative of _n_=3 independent replicates per condition. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; Scr, scrambled. *P<0.05.
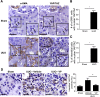
Figure 6.
Verteporfin treatment leads to reduced levels of YAP and TAZ in injured, fibrosing kidneys. (A) Mice undergoing UUO (_n_=4) or sham surgery (_n_=3) were euthanized 7 days postprocedure. Shown are representative images of serially cut sections immunostained for (left panels) _α_-SMA and (right panels) YAP/TAZ from (upper panels) a sham-operated kidney and (lower panels) an obstructed kidney. White arrows identify serially cut interstitial _α_-SMA+ cells, and black arrows identify serially cut interstitial cells. In UUO kidneys, _α_-SMA+ cells frequently exhibited nuclear YAP/TAZ staining. In sham-operated kidneys, no serially cut interstitial cells stained for both _α_-SMA and YAP/TAZ could be found. Scale bar, 80 _μ_m. (B) The number of cortical _α_-SMA+ cells and (C) the percentage of cortical interstitial cells with nuclear YAP/TAZ staining were quantified; t test was performed. In D, UUO mice were treated with (_n_=7) or without verteporfin (_n_=9) beginning immediately after surgery, with sham-operated mice as controls (_n_=6). Seven days later, reduced interstitial YAP/TAZ staining was seen in verteporfin-treated mice. Black arrows depict YAP/TAZ–stained interstitial cells. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 40 _μ_m. *P<0.05.
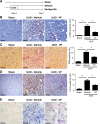
Figure 7.
Verteporfin significantly reduces renal fibrosis and myofibroblast accumulation after UUO. (A) Mice undergoing left-sided UUO were randomized to treatment with verteporfin (_n_=7) or vehicle (_n_=9) beginning immediately after surgery. Sham-operated mice served as healthy controls (_n_=6). Paraffin–embedded kidney tissue sections from verteporfin–treated UUO mice were stained with (B) an _α_-SMA antibody to identify interstitial myofibroblasts, (C) picrosirius red (PSR) to identify fibrillar collagen, and (D) a type 4 collagen (Col IV) antibody. Representative images from the different treatment groups are shown. Entire kidney sections (excluding the renal pelvis) were digitally analyzed for positive staining. Scale bar, 150 _μ_m. *P<0.05. (E) Paraffin–embedded kidney tissue sections were incubated with a digoxygenin-conjugated probe specific for CTGF mRNA. Blue staining depicts positive riboprobe staining. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 20 _μ_m.
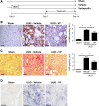
Figure 8.
Verteporfin arrests additional progression of established renal fibrosis after UUO. (A) Mice undergoing left-sided UUO were treated with verteporfin (_n_=10) or vehicle (_n_=7) beginning 7 days after surgery. Sham-operated mice served as healthy controls (_n_=4). Paraffin–embedded kidney tissue sections from verteporfin–treated UUO mice were stained with (B) an _α_-SMA antibody to identify interstitial myofibroblasts and (C) picrosirius red (PSR) to identify fibrillar collagen. Verteporfin treatment attenuated UUO-induced increases in _α_-SMA and fibrillar collagen staining. Representative images from the different treatment groups are shown. Entire kidney sections (excluding the renal pelvis) were digitally analyzed for positive staining. Scale bar, 50 _μ_m. (D) Paraffin–embedded kidney tissue sections were incubated with a digoxygenin-conjugated probe specific for CTGF mRNA. Blue staining depicts positive riboprobe staining. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 20 _μ_m. *P<0.05.
Similar articles
- Yap/Taz Deletion in Gli+ Cell-Derived Myofibroblasts Attenuates Fibrosis.
Liang M, Yu M, Xia R, Song K, Wang J, Luo J, Chen G, Cheng J. Liang M, et al. J Am Soc Nephrol. 2017 Nov;28(11):3278-3290. doi: 10.1681/ASN.2015121354. Epub 2017 Aug 2. J Am Soc Nephrol. 2017. PMID: 28768710 Free PMC article. - The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway.
Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. Varelas X, et al. Dev Cell. 2010 Dec 14;19(6):831-44. doi: 10.1016/j.devcel.2010.11.012. Dev Cell. 2010. PMID: 21145499 - YAP and TAZ are distinct effectors of corneal myofibroblast transformation.
Muppala S, Raghunathan VK, Jalilian I, Thomasy S, Murphy CJ. Muppala S, et al. Exp Eye Res. 2019 Mar;180:102-109. doi: 10.1016/j.exer.2018.12.009. Epub 2018 Dec 19. Exp Eye Res. 2019. PMID: 30578787 Free PMC article. - YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer.
Noguchi S, Saito A, Nagase T. Noguchi S, et al. Int J Mol Sci. 2018 Nov 20;19(11):3674. doi: 10.3390/ijms19113674. Int J Mol Sci. 2018. PMID: 30463366 Free PMC article. Review. - Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge.
Piersma B, Bank RA, Boersema M. Piersma B, et al. Front Med (Lausanne). 2015 Sep 3;2:59. doi: 10.3389/fmed.2015.00059. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26389119 Free PMC article. Review.
Cited by
- Adipose-derived mesenchymal stem cells inhibit hepatic stellate cells activation to alleviate liver fibrosis via Hippo pathway.
Liu H, Huang H, Liu Y, Yang Y, Deng H, Wang X, Zhou Z, Peng G, Jin S, Chen D, Zhong Z. Liu H, et al. Stem Cell Res Ther. 2024 Oct 24;15(1):378. doi: 10.1186/s13287-024-03988-7. Stem Cell Res Ther. 2024. PMID: 39449061 Free PMC article. - Acute contact with profibrotic macrophages mechanically activates fibroblasts via αvβ3 integrin-mediated engagement of Piezo1.
Ezzo M, Spindler K, Wang JB, Lee D, Pecoraro G, Cowen J, Pakshir P, Hinz B. Ezzo M, et al. Sci Adv. 2024 Oct 25;10(43):eadp4726. doi: 10.1126/sciadv.adp4726. Epub 2024 Oct 23. Sci Adv. 2024. PMID: 39441936 Free PMC article. - Endothelial YAP/TAZ activation promotes atherosclerosis in a mouse model of Hutchinson-Gilford progeria syndrome.
Barettino A, González-Gómez C, Gonzalo P, Andrés-Manzano MJ, Guerrero CR, Espinosa FM, Carmona RM, Blanco Y, Dorado B, Torroja C, Sánchez-Cabo F, Quintas A, Benguría A, Dopazo A, García R, Benedicto I, Andrés V. Barettino A, et al. J Clin Invest. 2024 Oct 1;134(22):e173448. doi: 10.1172/JCI173448. J Clin Invest. 2024. PMID: 39352768 Free PMC article. - Anti-fibrogenic effect of umbilical cord-derived mesenchymal stem cell-conditioned media in human esophageal fibroblasts.
Choi YJ, Kim JH, Lee Y, Pyeon HJ, Yoo IK, Yoo JH. Choi YJ, et al. Sci Rep. 2024 Sep 27;14(1):22233. doi: 10.1038/s41598-024-73091-7. Sci Rep. 2024. PMID: 39333200 Free PMC article. - Targeting Neuraminidase 4 Attenuates Kidney Fibrosis in Mice.
Xiao PT, Hao JH, Kuang YJ, Dai CX, Rong XL, Jiang LL, Xie ZS, Zhang L, Chen QQ, Liu EH. Xiao PT, et al. Adv Sci (Weinh). 2024 Oct;11(39):e2406936. doi: 10.1002/advs.202406936. Epub 2024 Aug 13. Adv Sci (Weinh). 2024. PMID: 39136142 Free PMC article.
References
- Attisano L, Wrana JL: Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002 - PubMed
- Shi Y, Massagué J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 - PubMed
- Leask A, Abraham DJ: TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources