Chemically induced skin carcinogenesis: Updates in experimental models (Review) - PubMed (original) (raw)
Review
Chemically induced skin carcinogenesis: Updates in experimental models (Review)
Monica Neagu et al. Oncol Rep. 2016 May.
Abstract
Skin cancer is one of the most common malignancies affecting humans worldwide, and its incidence is rapidly increasing. The study of skin carcinogenesis is of major interest for both scientific research and clinical practice and the use of in vivo systems may facilitate the investigation of early alterations in the skin and of the mechanisms involved, and may also lead to the development of novel therapeutic strategies for skin cancer. This review outlines several aspects regarding the skin toxicity testing domain in mouse models of chemically induced skin carcinogenesis. There are important strain differences in view of the histological type, development and clinical evolution of the skin tumor, differences reported decades ago and confirmed by our hands‑on experience. Using mouse models in preclinical testing is important due to the fact that, at the molecular level, common mechanisms with human cutaneous tumorigenesis are depicted. These animal models resemble human skin cancer development, in that genetic changes caused by carcinogens and pro‑inflammatory cytokines, and simultaneous inflammation sustained by pro‑inflammatory cytokines and chemokines favor tumor progression. Drugs and environmental conditions can be tested using these animal models. keeping in mind the differences between human and rodent skin physiology.
Figures
Figure 1
The cascade of acute and chronic skin inflammation. (A) The initial skin injury triggers intravascular processes that promote neutrophil adhesion and transmigration. Resident macrophages and mastocytes release pro-inflammatory factors and chemoattractants; (B) lymphocytes and monocytes have enhanced adhesion capacities and further transmigrate into the extravascular space. Transmigrated cells and resident macrophages secrete pro-inflammatory factors and chemoattractants, stimulating collagen production and perpetuating the inflammatory response (copyright from ref. 29).
Figure 2
Elements of acute and chronic inflammation that are linked to tumorigenesis. (A) T and B lymphocytes secrete factors that induce the M1 macrophage phenotype, promote the innate immune response, promote cytotoxic T lymphocyte (CTL)-mediated destruction, enhance the antigen-presenting capacity and increase natural killer (NK) cell activity. All these processes have a potent antitumorigenic effect; (B) T and B lymphocytes secrete factors that induce the M2 macrophage phenotype, enhance myeloid suppressor activity, reduce CTL activity, decrease the antigen-presenting capacity and increase NK cell activity. All these processes have a potent pro-tumorigenic effect (copyright from ref. 29). IL, interleukin; IFN-γ, interferon-γ; TGF-β, ransforming growth factor β; Ig, immunoglobulin.
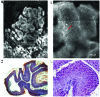
Figure 3
Reflectance confocal microscopy (RCM) and histopathological images of chemically induced skin tumors in C57BL/6 mice. (A) RCM mosaic showing a tumoral structure with an irregular architecture; (B and C) details of RCM image showing bright fiber-like structures (white arrowhead), large cells with dark nuclei (white arrows) and numerous small hyper-refractile structures (white asterisk); (D) poorly differentiated carcinoma expressing neuron-specific enolase, week cytokeratin expression, negative melanocyte markers (hematoxylin and eosin staining, original magnification, ×100); (E an F) histological details (hematoxylin and eosin staining, original magnification, ×400).
Figure 4
(a) Reflectance confocal microscopy (RCM) mosaic showing a tumoral structure with a multilobular architecture; (B) details of RCM image showing atypical cells (white asterisk) and blood vessels (red arrow); (C) cutaneous papillomas presenting intra-epithelial keratinocytes neoplasia I/II (hematoxylin and eosin staining, original magnification, ×100); (D) histological detail (hematoxylin and eosin staining, original magnification, ×400).
Similar articles
- VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis.
Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, Schneider N, Bry M, Detmar M. Alitalo AK, et al. Cancer Res. 2013 Jul 15;73(14):4212-21. doi: 10.1158/0008-5472.CAN-12-4539. Epub 2013 May 21. Cancer Res. 2013. PMID: 23695550 - Chemomodulatory Potential of Flaxseed Oil Against DMBA/Croton Oil-Induced Skin Carcinogenesis in Mice.
Sharma J, Singh R, Goyal PK. Sharma J, et al. Integr Cancer Ther. 2016 Sep;15(3):358-67. doi: 10.1177/1534735415608944. Epub 2015 Oct 5. Integr Cancer Ther. 2016. PMID: 26437861 Free PMC article. - Epidermal p65/NF-κB signalling is essential for skin carcinogenesis.
Kim C, Pasparakis M. Kim C, et al. EMBO Mol Med. 2014 Jul;6(7):970-83. doi: 10.15252/emmm.201303541. EMBO Mol Med. 2014. PMID: 24952939 Free PMC article. - Inflammation and skin cancer: old pals telling new stories.
Hensler S, Mueller MM. Hensler S, et al. Cancer J. 2013 Nov-Dec;19(6):517-24. doi: 10.1097/PPO.0000000000000010. Cancer J. 2013. PMID: 24270351 Review. - Cutaneous chemical carcinogenesis: past, present, and future.
Yuspa SH, Hennings H, Saffiotti U. Yuspa SH, et al. J Invest Dermatol. 1976 Jul;67(1):199-208. doi: 10.1111/1523-1747.ep12513040. J Invest Dermatol. 1976. PMID: 819592 Review.
Cited by
- Zinc oxide nanoparticles derived from Penicillium griseofulvum mitigate DMBA/TPA-promoted mice skin carcinogenesis by modulating NF-ĸB associated signalling.
Li Z, Wu F, Zhang Q. Li Z, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul 30. doi: 10.1007/s00210-024-03311-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39080011 - Fine scalpel surgery: preserving the dartos muscle in a patient with scrotal and perigenital giant Buschke-Löwenstein tumors.
Kordeva S, Pidakev I, Tchernev G. Kordeva S, et al. Wien Med Wochenschr. 2024 Nov;174(15-16):342-349. doi: 10.1007/s10354-024-01039-7. Epub 2024 Apr 8. Wien Med Wochenschr. 2024. PMID: 38587714 English. - Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment.
Udrea AM, Smarandache A, Dinache A, Mares C, Nistorescu S, Avram S, Staicu A. Udrea AM, et al. Pharmaceutics. 2023 Aug 11;15(8):2124. doi: 10.3390/pharmaceutics15082124. Pharmaceutics. 2023. PMID: 37631339 Free PMC article. Review. - Macrophage migration inhibitory factor (MIF) and its homolog D-dopachrome tautomerase (D-DT) are significant promotors of UVB- but not chemically induced non-melanoma skin cancer.
Huth S, Huth L, Heise R, Marquardt Y, Lopopolo L, Piecychna M, Boor P, Fingerle-Rowson G, Kapurniotu A, Yazdi AS, Bucala R, Bernhagen J, Baron JM. Huth S, et al. Sci Rep. 2023 Jul 18;13(1):11611. doi: 10.1038/s41598-023-38748-9. Sci Rep. 2023. PMID: 37464010 Free PMC article. - Husk-like Zinc Oxide Nanoparticles Induce Apoptosis through ROS Generation in Epidermoid Carcinoma Cells: Effect of Incubation Period on Sol-Gel Synthesis and Anti-Cancerous Properties.
Alhoqail WA, Alothaim AS, Suhail M, Iqbal D, Kamal M, Asmari MM, Jamal A. Alhoqail WA, et al. Biomedicines. 2023 Jan 23;11(2):320. doi: 10.3390/biomedicines11020320. Biomedicines. 2023. PMID: 36830857 Free PMC article.
References
- Bos JD. The skin as an organ of immunity. Clin Exp Immunol. 1997;107(suppl 1):3–5. - PubMed
- Bos JD. Skin immune system: Cutaneous immunology and clinical immunodermatology. 3rd edition. CRC Press; Boca Raton, FL: 2005. pp. 3–13.
- de Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D, Ioannides D, Aquilina S, Apap C, et al. EPIDERM Group: Known and potential new risk factors for skin cancer in European populations: A multicentre case-control study. Br J Dermatol. 2012;167(Suppl 2):1–13. doi: 10.1111/j.1365-2133.2012.11081.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical