Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution - PubMed (original) (raw)
. 2016 May 31;7(22):32100-12.
doi: 10.18632/oncotarget.8527.
Benjamin Le Calvé 1 2 3 4 5, Audrey Griveau 1 2 3 4, David Vindrieux 1 2 3 4, Clotilde Wiel 1 2 3 4, Magali Svrcek 7 8, Johann Gout 1 2 3 4, Lamia Azzi 9, Léa Payen 1 2 3 4 10, Jérôme Cros 11 12, Christelle de la Fouchardière 3, Pierre Dubus 9, Jérôme Guitton 4 10, Laurent Bartholin 1 2 3 4, Jean-Baptiste Bachet 13 14, David Bernard 1 2 3 4
Affiliations
- PMID: 27050073
- PMCID: PMC5078000
- DOI: 10.18632/oncotarget.8527
Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution
Benjamin Le Calvé et al. Oncotarget. 2016.
Abstract
Solid tumors often display chemotherapy resistance. Pancreatic ductal adenocarcinoma (PDAC) is the archetype of resistant tumors as current chemotherapies are inefficient. The tumor stroma and extracellular matrix (ECM) are key contributors to PDAC aggressiveness and to limiting the efficacy of chemotherapy. Lysyl oxidase (LOX) family members mediate collagen cross-linking and thus promote ECM stiffening. Our data demonstrate increased LOX, LOXL1, and LOXL2 expression in PDAC, and that the level of fibrillar collagen, which is directly dependent of LOX family activity, is an independent predictive biomarker of adjuvant "Gemcitabine-based chemotherapy" benefit. Experimentally in mice, increased LOX family activity through LOXL2 promotes chemoresistance. This effect of LOX family activity seems to be due to decreased gemcitabine intra-tumoral diffusion. This observation might be explained by increased fibrillar collagen and decreased vessel size observed in tumors with increased LOX family activity. In conclusion, our data support that LOX family activity is both a novel target to improve chemotherapy as well as a novel biomarker to predict gemcitabine benefit in PDAC. Beyond the PDAC, it is possible that targeting LOX family activity might improve efficacy of chemotherapies against different kinds of solid tumors.
Keywords: biomarker; chemoresistance; collagen; lysyl oxidase; survival.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
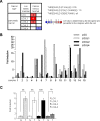
Figure 1. LOX activity increases in human PDAC
(A) Summary of studies comparing levels of LOX, LOXL1, LOXL2, LOXL3, and LOXL4 transcripts in PDAC versus normal-tissue counterparts, obtained using the Oncomine database with the following settings: threshold _P_-value: 0.05; fold change: 1.5; gene rank all. The number indicated the number of study showing the same differential. Red color indicated an increase in the tumor whereas blue color indicated a decrease. (B–C) RNAs were extracted from both pancreatic normal and tumor tissues from the same patients and LOX, LOXL1, LOXL2, LOXL3, and LOXL4 transcript levels were assayed by qRT-PCR and normalized against HPRT1. LOXL3 was not detected. (B) Results are presented for each patient. (C) Results are presented as means ± SEM of LOX, LOXL1, LOXL2, and LOXL4 induction. _P_-values were determined with the Mann-Whitney U test.
Figure 2. Modulating LOX family activity impacts the response to gemcitabine treatment
One million Colo-357/Luc pancreatic cancer cells were orthotopically grafted into 6-week-old nude mice. The mice were left untreated or received IV injections of 40 mg/kg gemcitabine, alone or with LOXL2 or LOXL2 + LOXi injected IP when the tumors reached a mean luminescence of 800cts/s (n = 7 per group). Sixty-three days after the graft, (A) part of the tumors was used to measure LOX family activity or (B) the tumors were fixed, embedded, and tissue sections were stained with picrosirius red. Fibrillar collagen was analyzed by microscopy under polarized light. Values are means ± SEM. (C–D) Sixty-three days after grafting, tumor luminescence was measured. (C) Representative images of tumor development after 63 days are displayed for each condition; (D) luminescence is expressed as an integration of the average brightness/pixel unit (cts/s). (E) Immunohistochemical staining for the proliferation marker Ki-67 was performed. Representative photographs for each experimental condition are displayed. Ki-67-positive cells were counted in at least 25 fields. (F) A TUNEL cell death assay was performed. Representative photographs for each experimental condition are displayed. TUNEL-positive cells were counted in at least 15 fields. (A–E) Values are means ± SEM and the statistical test used was Mann-Whitney U.
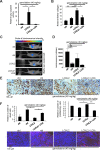
Figure 3. LOX family activity decreases gemcitabine distribution and favors vessels collapse
(A–B) One hour before the endpoint of the orthotopic experiment described in Figure 3, 40 mg/kg gemcitabine was injected. At euthanasia, plasma and tumors of from the groups having received gemcitabine alone or in combination with LOXL2 or LOXL2+LOXi were removed for measurement of the gemcitabine concentration. The gemcitabine concentrations were determined (A) in the plasma and (B) in the tumor for each group and the results are expressed in μg of gemcitabine per mg protein ± SEM. The statistical test used was Mann-Whitney U. (C–D) Orthotopic tumors as described in Figure 3 were analyzed by immunohistochemical staining for CD31, an endothelial cell marker. (C) Vessel size was calculated with ImageJ software and (D) representative photographs are displayed. Values are means ± SEM and the statistical test used was Mann-Whitney U.
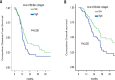
Figure 4. High fibrillar collagen level group displays lower survival during adjuvant gemcitabine-based therapy
Level of fibrillar collagen was determined by calculating the ratio between fibrillar collagen and the quantity of collagen. Patients having received an adjuvant Gemcitabine-based chemotherapy were analyzed and samples were split in 2 groups, half of having the highest ratio and half having the lowest ratio. Kaplan Meier analyses were performed and represented (A) the disease free-survival and (B) the overall survival. P values were calculated using a log rank test.
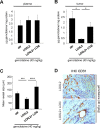
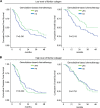
Figure 5. Fibrillar collagen level is a predictive factor for gemcitabine benefit
Level of fibrillar collagen was determined by calculating the ratio between fibrillar collagen and the quantity of collagen. (A) Patients having a poor level of fibrillar collagen (mean 50% of the patients with the lowest collagen organization/quantity ratio) were analyzed and samples were split in 2 groups, having received or not an adjuvant gemcitabine-based chemotherapy. Kaplan Meier analyses were performed and represented the disease free-survival (left panel) and the overall survival (right panel). P values were calculated using a log rank test. (B) A similar analysis was performed in patients having a high level of fibrillar collagen (mean 50% of the patients with the lowest collagen organization/quantity ratio).
Similar articles
- S100A14 promotes progression and gemcitabine resistance in pancreatic cancer.
Zhu H, Gao W, Li X, Yu L, Luo D, Liu Y, Yu X. Zhu H, et al. Pancreatology. 2021 Apr;21(3):589-598. doi: 10.1016/j.pan.2021.01.011. Epub 2021 Jan 22. Pancreatology. 2021. PMID: 33579599 - Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2.
Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ, Munshi HG. Dangi-Garimella S, et al. Cancer Res. 2011 Feb 1;71(3):1019-28. doi: 10.1158/0008-5472.CAN-10-1855. Epub 2010 Dec 8. Cancer Res. 2011. PMID: 21148071 Free PMC article. - TRIM11 suppresses ferritinophagy and gemcitabine sensitivity through UBE2N/TAX1BP1 signaling in pancreatic ductal adenocarcinoma.
Shang M, Weng L, Xu G, Wu S, Liu B, Yin X, Mao A, Zou X, Wang Z. Shang M, et al. J Cell Physiol. 2021 Oct;236(10):6868-6883. doi: 10.1002/jcp.30346. Epub 2021 Feb 25. J Cell Physiol. 2021. PMID: 33629745 - Gemcitabine resistance in pancreatic ductal adenocarcinoma.
Binenbaum Y, Na'ara S, Gil Z. Binenbaum Y, et al. Drug Resist Updat. 2015 Nov;23:55-68. doi: 10.1016/j.drup.2015.10.002. Epub 2015 Nov 3. Drug Resist Updat. 2015. PMID: 26690340 Review. - Mechanisms of drug resistance of pancreatic ductal adenocarcinoma at different levels.
Du J, Gu J, Li J. Du J, et al. Biosci Rep. 2020 Jul 31;40(7):BSR20200401. doi: 10.1042/BSR20200401. Biosci Rep. 2020. PMID: 32677676 Free PMC article. Review.
Cited by
- Mechano-therapeutics: Targeting Mechanical Signaling in Fibrosis and Tumor Stroma.
Tschumperlin DJ, Lagares D. Tschumperlin DJ, et al. Pharmacol Ther. 2020 Aug;212:107575. doi: 10.1016/j.pharmthera.2020.107575. Epub 2020 May 11. Pharmacol Ther. 2020. PMID: 32437826 Free PMC article. Review. - Reciprocal Regulation of Cancer-Associated Fibroblasts and Tumor Microenvironment in Gastrointestinal Cancer: Implications for Cancer Dormancy.
Cheng SH, Chiou HC, Wang JW, Lin MH. Cheng SH, et al. Cancers (Basel). 2023 Apr 27;15(9):2513. doi: 10.3390/cancers15092513. Cancers (Basel). 2023. PMID: 37173977 Free PMC article. Review. - Targets in the Tumour Matrisome to Promote Cancer Therapy Response.
Jalil SMA, Henry JC, Cameron AJM. Jalil SMA, et al. Cancers (Basel). 2024 May 11;16(10):1847. doi: 10.3390/cancers16101847. Cancers (Basel). 2024. PMID: 38791926 Free PMC article. Review. - Roles of Lysyl Oxidase Family Members in the Tumor Microenvironment and Progression of Liver Cancer.
Lin HY, Li CJ, Yang YL, Huang YH, Hsiau YT, Chu PY. Lin HY, et al. Int J Mol Sci. 2020 Dec 21;21(24):9751. doi: 10.3390/ijms21249751. Int J Mol Sci. 2020. PMID: 33371259 Free PMC article. Review. - LOXL1 modulates the malignant progression of colorectal cancer by inhibiting the transcriptional activity of YAP.
Hu L, Wang J, Wang Y, Wu L, Wu C, Mao B, Maruthi Prasad E, Wang Y, Chin YE. Hu L, et al. Cell Commun Signal. 2020 Sep 10;18(1):148. doi: 10.1186/s12964-020-00639-1. Cell Commun Signal. 2020. PMID: 32912229 Free PMC article.
References
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
- Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–97. - PubMed
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
- Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249–56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials