Using Drosophila to discover mechanisms underlying type 2 diabetes - PubMed (original) (raw)
Review
Using Drosophila to discover mechanisms underlying type 2 diabetes
Ronald W Alfa et al. Dis Model Mech. 2016 Apr.
Abstract
Mechanisms of glucose homeostasis are remarkably well conserved between the fruit flyDrosophila melanogasterand mammals. From the initial characterization of insulin signaling in the fly came the identification of downstream metabolic pathways for nutrient storage and utilization. Defects in these pathways lead to phenotypes that are analogous to diabetic states in mammals. These discoveries have stimulated interest in leveraging the fly to better understand the genetics of type 2 diabetes mellitus in humans. Type 2 diabetes results from insulin insufficiency in the context of ongoing insulin resistance. Although genetic susceptibility is thought to govern the propensity of individuals to develop type 2 diabetes mellitus under appropriate environmental conditions, many of the human genes associated with the disease in genome-wide association studies have not been functionally studied. Recent advances in the phenotyping of metabolic defects have positionedDrosophilaas an excellent model for the functional characterization of large numbers of genes associated with type 2 diabetes mellitus. Here, we examine results from studies modeling metabolic disease in the fruit fly and compare findings to proposed mechanisms for diabetic phenotypes in mammals. We provide a systematic framework for assessing the contribution of gene candidates to insulin-secretion or insulin-resistance pathways relevant to diabetes pathogenesis.
Keywords: Diabetes; Drosophila; Insulin resistance; Insulin-like peptides.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests
The authors declare no competing or financial interests.
Figures
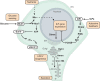
Fig. 1.
Genetic pathways to glucose intolerance. The diagram depicts a simplified framework for organizing the molecular mechanisms underlying diabetic phenotypes in model organisms and humans. Glucose intolerance (hyperglycemia) and type 2 diabetic phenotypes result from the combination of insulin resistance and functional insulin deficiency due to inadequate compensation, i.e. inadequate upregulation of insulin output. Insulin resistance results from primary defects (primary mechanisms) in insulin/IGF-like signaling (IIS) or through secondary mechanisms that prevent insulin from binding to its receptor or disrupt effectors downstream of IIS. Conversely, mutations that cause insulin deficiency phenotypes affect genes involved in the secretion of insulins (intrinsic mechanisms) or the non-autonomous modulation of insulin production or secretion (extrinsic mechanisms).
Fig. 2.
Intrinsic regulators of insulin-like peptide output in Drosophila. Schematic of a Drosophila insulin-producing cell (IPC) cell body and pathways involved in insulin-like peptide (ILP) production and secretion, including: transcription, translation, processing and secretion of ILPs. Dietary nutrients such as protein or carbohydrates control transcription of ILPs through unknown mechanisms, which might involve the glucose-responsive transcription factor Mio or IIS feedback signaling through FOXO. ILP expression also seems to be under autocrine control through insulin/IGF-like signaling (IIS). In response to IIS, FOXO is phosphorylated and retained in the cytoplasm, unable to activate expression of ILPs. A number of genes are important for the processing and packaging of ILPs into large dense core vesicles (LDVCs), including: Dimmed (Dimm), Rab1 GTPase, Amontillado (amon) and Unc-104 ortholog (Unc-104). In stimulus-secretion coupling, glucose enters the cell through Glut1 and is acted on by an unknown hexokinase (‘Hex?’) to generate ATP. ATP binds to the KATP-channel subunit Sur and depolarizes the membrane (ΔVm) by decreasing conductance through an inward rectifying potassium channel (Ir). Fusion of LDCVs and insulin secretion occurs through activation of unknown voltage-gated calcium channels (VGCCs). See main text for details. ER, endoplasmic reticulum. Limited data exists for pathways indicated by hatched lines.
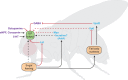
Fig. 3.
Extrinsic regulators of insulin-like peptide output in Drosophila. Schematic of an adult Drosophila, depicting extrinsic pathways that regulate insulin-like peptide (ILP) output from insulin-producing cells (IPCs). The location of IPCs in the central brain is indicated by the green box (ILP output). Neuromodulators and neuropeptide systems that modulate ILP secretion in the central brain are indicated in magenta text. Peripheral modulators of ILP secretion are indicated in blue text. Dietary sugar controls ILP output through the CC-cell-derived decretin hormone Limostatin (Lst), the fat body nutrient sensor and through unknown direct (hatched black lines) and incretin-like mechanisms. The fat body acts as a nutrient sensor to remotely control ILP output through secreted factors Unpaired2 (Upd2) and Ilp6. The myokinin Myoglianin (Myo) is secreted from muscle tissue and mediates inhibitory control over ILP output. sNPF, short Neuropeptide F; 5-HT, serotonin neurons; AstA, Allatostatin A. Positive pathways are shown by black arrows; inhibitory pathways are shown by red lines. Limited data exists for pathways indicated by hatched lines. See main text for additional discussion.
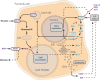
Fig. 4.
Insulin resistance and feedback in the Drosophila fat body. Schematic depiction of a Drosophila fat body cell illustrating gene products that might contribute to primary (blue text) and secondary (magenta text) mechanisms of insulin resistance. Primary mechanisms: alterations of insulin receptor (InR) or any downstream components of the insulin/IGF-like signaling (IIS) pathway lead to insulin resistance. Target of rapamycin (TOR) and Jun-N-terminal kinase (JNK) pathways are proposed to negatively regulate IIS signaling and thus contribute to insulin resistance. Lipid accumulation [which occurs when flies are fed a high-sugar diet (HSD); see main text] leads to PKC activation, which contributes to insulin resistance through negative feedback on IIS. Secondary mechanisms: the secreted insulin-binding proteins Imp-L2, Secreted decoy of InR (SDR) and Acid-labile subunit (ALS) cause insulin resistance by interfering with the binding of insulin to its receptor. IIS triggers membrane localization of an unknown Drosophila glucose transporter (Glut?) through membrane fusion of lipid rafts to facilitate removal of hemolymph glucose, and defects in this glucose transporter or its trafficking might lead to insulin resistance. AKH stimulates lipolysis (involving AkhR and PKA), and defects in this pathway lead to obesity. AKH also increases hemolymph glucose through unknown mechanisms that likely involve gluconeogenesis and breakdown of glycogen, and might contribute to hyperglycemia in diabetic states. Fat body secreted factors (endocrine, lower right) Unpaired2 (Upd2) and insulin-like peptide 6 (Ilp6) might contribute to insulin resistance in certain nutritional states. PKA, Protein kinase A; AKH, Adipokinetic hormone; AkhR, Adipokinetic hormone receptor; ER, endoplasmic reticulum; SVs, secretory vesicles; MT, mitochondrion. Positive pathways are shown by black arrows; inhibitory pathways are shown by red lines. Limited data exists for pathways indicated by hatched lines. See main text for additional discussion.
Similar articles
- A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion.
Park S, Alfa RW, Topper SM, Kim GE, Kockel L, Kim SK. Park S, et al. PLoS Genet. 2014 Aug 7;10(8):e1004555. doi: 10.1371/journal.pgen.1004555. eCollection 2014 Aug. PLoS Genet. 2014. PMID: 25101872 Free PMC article. - Drosophila Models to Investigate Insulin Action and Mechanisms Underlying Human Diabetes Mellitus.
Inoue YH, Katsube H, Hinami Y. Inoue YH, et al. Adv Exp Med Biol. 2018;1076:235-256. doi: 10.1007/978-981-13-0529-0_13. Adv Exp Med Biol. 2018. PMID: 29951823 Review. - An in vivo screen for neuronal genes involved in obesity identifies Diacylglycerol kinase as a regulator of insulin secretion.
Trinh I, Gluscencova OB, Boulianne GL. Trinh I, et al. Mol Metab. 2019 Jan;19:13-23. doi: 10.1016/j.molmet.2018.10.006. Epub 2018 Oct 19. Mol Metab. 2019. PMID: 30389349 Free PMC article. - The water extract of Potentilla discolor Bunge (PDW) ameliorates high-sugar diet-induced type II diabetes model in Drosophila melanogaster via JAK/STAT signaling.
Li Y, Wang J, Xu Y, Meng Q, Wu M, Su Y, Miao Y, Wang Y. Li Y, et al. J Ethnopharmacol. 2023 Nov 15;316:116760. doi: 10.1016/j.jep.2023.116760. Epub 2023 Jun 8. J Ethnopharmacol. 2023. PMID: 37301307
Cited by
- Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms.
Mascolo E, Vernì F. Mascolo E, et al. Int J Mol Sci. 2020 May 23;21(10):3669. doi: 10.3390/ijms21103669. Int J Mol Sci. 2020. PMID: 32456137 Free PMC article. Review. - Taking Stock of the Drosophila Research Ecosystem.
Bilder D, Irvine KD. Bilder D, et al. Genetics. 2017 Jul;206(3):1227-1236. doi: 10.1534/genetics.117.202390. Genetics. 2017. PMID: 28684603 Free PMC article. Review. - Systemic corazonin signalling modulates stress responses and metabolism in Drosophila.
Kubrak OI, Lushchak OV, Zandawala M, Nässel DR. Kubrak OI, et al. Open Biol. 2016 Nov;6(11):160152. doi: 10.1098/rsob.160152. Open Biol. 2016. PMID: 27810969 Free PMC article. - Modeling Obesity-Associated Ovarian Dysfunction in Drosophila.
Liu H, Li J, Chang X, He F, Ma J. Liu H, et al. Nutrients. 2022 Dec 16;14(24):5365. doi: 10.3390/nu14245365. Nutrients. 2022. PMID: 36558524 Free PMC article. - The Glucagon-Like Adipokinetic Hormone in Drosophila melanogaster - Biosynthesis and Secretion.
Hughson BN. Hughson BN. Front Physiol. 2021 Dec 23;12:710652. doi: 10.3389/fphys.2021.710652. eCollection 2021. Front Physiol. 2021. PMID: 35002748 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases