MiR-299-5p regulates apoptosis through autophagy in neurons and ameliorates cognitive capacity in APPswe/PS1dE9 mice - PubMed (original) (raw)
MiR-299-5p regulates apoptosis through autophagy in neurons and ameliorates cognitive capacity in APPswe/PS1dE9 mice
Yueqi Zhang et al. Sci Rep. 2016.
Abstract
Abnormalities of autophagy can result in neurodegenerative disorders such as Alzheimer's disease (AD). Nevertheless, the regulatory mechanisms of autophagy in AD are not well understood. Here, we describe our findings that microRNA (miR)-299-5p functions as an autophagy inhibitor by suppressing Atg5 and antagonizing caspase-dependent apoptosis. We observed decreased levels of miR-299-5p both in primary neurons under conditions of starvation and in hippocampi of APPswe/PS1dE9 mice. Additionally, low levels of miR-299-5p were observed in cerebrospinal fluid of AD patients. MiR-299-5p treatment resulted in attenuation of Atg5 and autophagy in primary neurons from APPswe/PS1dE9 mice, N2a cells and SH-SY5Y cells, whereas antagomiR-299-5p enhanced autophagy. Atg5 was verified as a direct target of miR-299-5p by dual luciferase reporter assays. Furthermore, transfection of miR-299-5p into primary hippocampal neurons caused the attenuation of caspase-mediated apoptosis, which was reversed upon starvation-induced autophagy. Inhibition of autophagy by shRNA knockdown of LC3β reduced apoptotic neuron death induced by antagomiR-299-5p. Injection of agomiR-299-5p into the cerebral ventricles of AD mice inhibited both autophagy and apoptosis and also improved the cognitive performance of mice. Overall, our results suggest that miR-299-5p modulates neuron survival programs by regulating autophagy. Thus, miR-299-5p serves as a potential neuroprotective factor in AD.
Figures
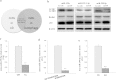
Figure 1. Decreased levels of miR-299-5p in autophagy and AD.
(a) Venn diagram showing the AD-associated miRs (light) and the miRs that were altered under starvation conditions (dark). The overlapping 3 miRs were considered as potential regulators both in autophagy and in AD. (b) The 3 miRs or scrambled miRs (Scr-miR) were transfected into primary neurons for 24 h, and then autophagy related proteins were analyzed by western blotting (n = 3). β-actin was used as a loading control. (c) RT-PCR confirmed that mmu-miR-299-5p levels were lower in 9-month-old APPswe/PS1dE9 mice (Tg) than C57 mice (n = 4 per group). SnRNA U6 was used as an internal control. (d) RT-PCR analysis of endogenous miR-299-5p levels under no starvation or starvation (4 h) conditions. **P < 0.01. Bars indicated mean ± SD of 3 independent experiments. (e) RT-PCR showed downregulation of miR-299-5p in CSF of AD patients (n = 6 per group). **P < 0.01.
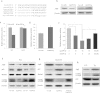
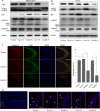
Figure 2. MiR-299-5p affects Atg5 levels directly and alters the levels of other autophagy-related proteins.
(a) Conservation of miR-299-5p and the predicted binding sequences between miR-299-5p and target sites in the Atg5 3′UTR. Matches within the seed sequence are indicated. The mutated sequences are showed as lower case letters. (b) Primary hippocampal neurons from AD mice were transfected with agomiR-299-5p (Ago299-5p) or antagomiR-299-5p (AM299-5p). The levels of Atg5 protein were analyzed by western blotting. (c) Luciferase vectors were generated by inserting the wild-type (WT) or mutated (MUT) 3′UTR fragments of Atg5 position 489–498 into pmirGLO plasmid. Normalized luciferase activity in lysates from Hela cells were assayed 24 h after transfection (mean ± SD of independent experiments, n = 3, *p < 0.05. #represents no significance). (d) AM299-5p increased luciferase activity when co-transfected with vectors containing a WT fragment (mean ± SD of independent experiments, n = 3, *p < 0.05). (e) Transfection with Ago299-5p decreased the luciferase activity of vectors containing WT fragment in a dose-dependent manner, and co-transfection with AM299-5p dose-dependently counteracted the effects of Ago299-5p in a dose-dependent manner (mean ± SD of independent experiments, n = 3, *p < 0.05). (f) In Neuro-2a (N2a) cells, Atg5 protein levels were reduced by transfection with Ago299-5p and increased by transfection with AM299-5p. Other autophagy-related proteins (LC3β and p62) were also modulated (n = 3). (g) In SH-SY5Y cells, transfection with Ago299-5p (40 nM) modulated Atg5, LC3β and p62 protein levels, and the effects were attenuated by co-transfection with AM299-5p (40 nM, n = 3). (h) Atg5 and LC3β protein levels in hippocampi of 9-month-old APPswe/PS1dE9 mice (Tg) and C57 mice (n = 4 per group).
Figure 3. Assessment of autophagy in miR-299-5p (Ago299-5p) or antagomiR-299-5p (AM299-5p)-overexpressing cells.
(a) Cy3-labeled Ago299-5p blocked GFP-LC3 puncta formation, and AM299-5p promoted puncta formation in Neuro-2a cells (n = 100). Scale bar = 25 μm. (b) Quantitative analysis of the experiments in panel (a) (mean ± SD of independent experiments, n = 4, *p < 0.05). (c) Representative TEM images of AVs in control and miR-299-5p/AM299-5p-overexpressing primary hippocampal neurons from AD mice. Scale bar = 2 μm.
Figure 4. MiR-299-5p regulates autophagy and apoptosis in primary hippocampal neurons.
(a) Primary hippocampal neurons were treated with Ago299-5p/AM299-5p or their scrambled controls at 40 μM for the indicated time and then cell viability was measured by MTT assay (mean ± SD of independent experiments, n = 3, *p < 0.05, **p < 0.01). (b) Top: EBSS culture accelerated apoptosis of Ago299-5p rescued neurons; Bottom: LC3β knockdown attenuated AM299-5p-induced apoptotic cell death. Primary neurons were pretreated for 20 h with Ago299-5p then cultured with EBSS for 4 h and stained with FITC-conjugated Annexin V/PI for flow cytometry. Primary neurons with or without stable LC3β knock down were treated with AM299-5p for 24 h followed by Annexin V/PI staining and flow cytometry. Right: The bar graph indicates the percentage of apoptotic neurons. The Annexin V positive and PI negative (early apoptotic, EA; light bars) and Annexin V and PI double positive (late apoptotic, LA, dark bars) apoptotic neurons are shown (mean ± SD of independent experiments, n = 3, *p < 0.05, #represents no significance). (c) Primary neurons were transfected with scrambled control or Ago299-5p for 24 h. or primary neurons were transfected with Ago299-5p for 20 h then switched to EBSS medium for an additional 4 h (totally 24 h). Western blot analysis was performed with antibodies against caspase-3, -8, Atg5, LC3β, p62 or Beclin1. β-actin was tested as loading control (n = 3). (d) Primary neurons with or without stable LC3β knockdown were treated with AM299-5p or its scrambled control for 24 h. Western blot analysis was performed using the indicated antibodies (n = 3). (e) Neurons were treated as in (c,d); caspase-3 activity was then measured using a commercial kit. Data are normalized to scrambled controls (mean ± SD of independent experiments, n = 3, *p < 0.05, **p < 0.01). (f) Neurons were treated as in (c and d); caspase-8 activity was then measured using a commercial kit. Data are normalized to scrambled controls (mean ± SD of independent experiments, n = 3, *p < 0.05).
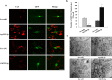
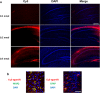
Figure 5. After injection, Cy3-labeled Ago299-5p is distributed throughout the brain tissues.
(a) 9-month-old APPswe/PS1dE9 mice were injected with different doses of Cy3-labeled Ago299-5p into the third ventricle (n = 3). Fluorescence of Cy3 was disseminated throughout the hippocampi and surrounding tissues after injection with 0.5 nmol for 24 h. Scale bar = 250 μm. (b) Cy3-labeled Ago299-5p was absorbed into MAP2-positive neurons and GFAP-positive glial cells. Scale bar = 25 μm.
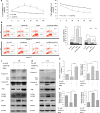
Figure 6. Therapeutic effects on protein levels.
(a) The alteration of protein levels in the hippocampus after treatment with Ago299-5p (n = 5 per group). (b) The alteration of protein levels in cortex after treatment with Ago299-5p (n = 5 per group). (c) The fluorescence intensity of cleaved caspase-3 in the hippocampus was weakened with the decreasing intensity of Atg5 after treatment with Ago299-5p (n = 5 per group). Scale bar = 250 μm. (d) Apoptosis examination was performed by TUNEL assay, TUNEL positive cells were marked with arrows. Scale bar = 50 μm. Representative TUNEL assay images were captured from the boxed region. (e) The percentage of positive cells was calculated as the apoptosis rate (number of positive cells/total number of cells × 100%) (mean ± SD, n = 5 per group, *p < 0.05, #represents no significance).
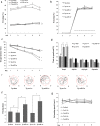
Figure 7. Behavior changes of APPswe/PS1dE9 mice after treatment with Ago299-5p.
(a) In a contextual fear conditioning test, the freezing time for APPswe/PS1dE9 mice treated with Ago299-5p was higher than for Scr-miR-treated transgenic mice (Tg-ctr) or sham untreated mice (Tg-sham), both at 1 and 3 weeks after injection. (b) In the cued conditioning test, there was no significant difference between these five groups. The sound cue intervention is showed as dotted line. (c) In the MWM test, the escape latency to reach the hidden platform during the acquisition phase was determined over 5 days. (d) The percentage of time mice spent in the four quadrants during the probe trial of the MWM test. (e) Representative swimming trajectories in the probe trial. (f) Passing times in the probe trial. (g) The average swimming speed during the water maze training (mean ± SD, n = 5 per group, *p < 0.05 compared with control group, #represents no significance).
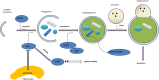
Figure 8. Model of miR-299-5p effect on autophagy and apoptosis in AD.
MiR-299-5p suppresses autophagy by targeting Atg5, which functions at the stage of elongation of membranes. Atg5 induces caspase activation following cleavage of Atg5 by calpain. Cleaved caspase-3 accumulates in autophagosomes. In addition caspase-8 may be cleaved in a similar fashion.
Similar articles
- Inhibition of miR-331-3p and miR-9-5p ameliorates Alzheimer's disease by enhancing autophagy.
Chen ML, Hong CG, Yue T, Li HM, Duan R, Hu WB, Cao J, Wang ZX, Chen CY, Hu XK, Wu B, Liu HM, Tan YJ, Liu JH, Luo ZW, Zhang Y, Rao SS, Luo MJ, Yin H, Wang YY, Xia K, Tang SY, Xie H, Liu ZZ. Chen ML, et al. Theranostics. 2021 Jan 1;11(5):2395-2409. doi: 10.7150/thno.47408. eCollection 2021. Theranostics. 2021. PMID: 33500732 Free PMC article. - miR-200a-3p promotes b-Amyloid-induced neuronal apoptosis through down-regulation of SIRT1 in Alzheimer's disease.
Zhang QS, Liu W, Lu GX. Zhang QS, et al. J Biosci. 2017 Sep;42(3):397-404. doi: 10.1007/s12038-017-9698-1. J Biosci. 2017. PMID: 29358553 - MicroRNA-20b-5p aggravates neuronal apoptosis induced by β-Amyloid via down-regulation of Ras homolog family member C in Alzheimer's disease.
Tian Z, Dong Q, Wu T, Guo J. Tian Z, et al. Neurosci Lett. 2021 Jan 18;742:135542. doi: 10.1016/j.neulet.2020.135542. Epub 2020 Dec 2. Neurosci Lett. 2021. PMID: 33278507 - Physical Exercise Ameliorates the Cognitive Function and Attenuates the Neuroinflammation of Alzheimer's Disease via miR-129-5p.
Li Z, Chen Q, Liu J, Du Y. Li Z, et al. Dement Geriatr Cogn Disord. 2020;49(2):163-169. doi: 10.1159/000507285. Epub 2020 May 20. Dement Geriatr Cogn Disord. 2020. PMID: 32434194 - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
- The Potential of Targeting Autophagy-Related Non-coding RNAs in the Treatment of Alzheimer's and Parkinson's Diseases.
Talebi Taheri A, Golshadi Z, Zare H, Alinaghipour A, Faghihi Z, Dadgostar E, Tamtaji Z, Aschner M, Mirzaei H, Tamtaji OR, Nabavizadeh F. Talebi Taheri A, et al. Cell Mol Neurobiol. 2024 Mar 10;44(1):28. doi: 10.1007/s10571-024-01461-w. Cell Mol Neurobiol. 2024. PMID: 38461204 Free PMC article. Review. - NcRNAs: A synergistically antiapoptosis therapeutic tool in Alzheimer's disease.
Li L, Jin M, Tan J, Xiao B. Li L, et al. CNS Neurosci Ther. 2024 Apr;30(4):e14476. doi: 10.1111/cns.14476. Epub 2023 Sep 22. CNS Neurosci Ther. 2024. PMID: 37735992 Free PMC article. Review. - Modulation of microRNAs through Lifestyle Changes in Alzheimer's Disease.
Pinto-Hernandez P, Castilla-Silgado J, Coto-Vilcapoma A, Fernández-Sanjurjo M, Fernández-García B, Tomás-Zapico C, Iglesias-Gutiérrez E. Pinto-Hernandez P, et al. Nutrients. 2023 Aug 23;15(17):3688. doi: 10.3390/nu15173688. Nutrients. 2023. PMID: 37686720 Free PMC article. Review. - Peroxiredoxin 2 Ameliorates AβO-Mediated Autophagy by Inhibiting ROS via the ROS-NRF2-p62 Pathway in N2a-APP Swedish Cells.
Jin W, Kam MK, Lee SW, Park YH, Lee HJ, Lee DS. Jin W, et al. Antioxidants (Basel). 2022 Sep 23;11(10):1889. doi: 10.3390/antiox11101889. Antioxidants (Basel). 2022. PMID: 36290612 Free PMC article. - MicroRNA silencing: A promising therapy for Alzheimer's disease.
Chauhan NB. Chauhan NB. Neurosci Chron. 2020;1(1):11-15. doi: 10.46439/neuroscience.1.004. Neurosci Chron. 2020. PMID: 35991586 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources