Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis - PubMed (original) (raw)
Review
Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis
Cheng-Yen Kao et al. Biomed J. 2016 Feb.
Abstract
Helicobacter pylori pathogenesis and disease outcomes are mediated by a complex interplay between bacterial virulence factors, host, and environmental factors. After H. pylori enters the host stomach, four steps are critical for bacteria to establish successful colonization, persistent infection, and disease pathogenesis: (1) Survival in the acidic stomach; (2) movement toward epithelium cells by flagella-mediated motility; (3) attachment to host cells by adhesins/receptors interaction; (4) causing tissue damage by toxin release. Over the past 20 years, the understanding of H. pylori pathogenesis has been improved by studies focusing on the host and bacterial factors through epidemiology researches and molecular mechanism investigations. These include studies identifying the roles of novel virulence factors and their association with different disease outcomes, especially the bacterial adhesins, cag pathogenicity island, and vacuolating cytotoxin. Recently, the development of large-scale screening methods, including proteomic, and transcriptomic tools, has been used to determine the complex gene regulatory networks in H. pylori. In addition, a more available complete genomic database of H. pylori strains isolated from patients with different gastrointestinal diseases worldwide is helpful to characterize this bacterium. This review highlights the key findings of H. pylori virulence factors reported over the past 20 years.
Keywords: Gastric cancer; Helicobacter pylori; Pathogenesis; Virulence factor.
Copyright © 2016 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Figures
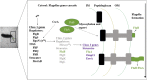
Fig. 1
Schematic diagram of Helicobacter pylori infection and pathogenesis. The urease activity and flagella-mediated motility of H. pylori facilitate its survival and movement toward the lower mucus gel above the epithelium, followed by several adhesins, including blood-antigen binding protein A, sialic acid-binding adhesin, and other outer membrane proteins interacting with receptors on the host epithelium cells. After successful colonization, toxins, including cytotoxin-associated gene A, and vacuolating cytotoxin A, are involved in damage of host tissue and intracellular replication.
Fig. 2
Current model of the flagellar transcriptional regulatory cascade for Helicobacter pylori flagellar biosynthesis. These genes are color-coded on the basis of their classification in the transcriptional regulatory cascade: black (class 1 genes), gray (class 2 genes), and purple (class 3 genes). Class 1 flagellar genes comprise most of the major regulatory genes of the flagellar system, including σ54 (RpoN), and FlgR. Formation of the flagellar T3SS has been proposed to create a signal detected by the FlgS sensor kinase, resulting in autophosphorylation of the protein. Phosphotransfer to the FlgR response regulator activates the protein, allowing for interactions with and stimulation of σ54. The expression of σ54 is positively regulated by CsrA through unclear mechanism. Alternative sigma factor σ28 (FliA) and structural proteins, including the minor flagellin FlaB, belong to Class 2 flagellar genes and are under the control of σ54. The T3SS facilitates the ordered secretion of the class 2 rod, ring, and hook proteins. The class 3 genes include flaA, which encodes the major flagellin, and those for other minor filament proteins, under the control of σ28.
Similar articles
- Pathogenesis of Helicobacter pylori infection.
Cid TP, Fernández MC, Benito Martínez S, Jones NL. Cid TP, et al. Helicobacter. 2013 Sep;18 Suppl 1:12-7. doi: 10.1111/hel.12076. Helicobacter. 2013. PMID: 24011239 Review. - Helicobacter pylori virulence genes.
Šterbenc A, Jarc E, Poljak M, Homan M. Šterbenc A, et al. World J Gastroenterol. 2019 Sep 7;25(33):4870-4884. doi: 10.3748/wjg.v25.i33.4870. World J Gastroenterol. 2019. PMID: 31543679 Free PMC article. Review. - Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders.
Nejati S, Karkhah A, Darvish H, Validi M, Ebrahimpour S, Nouri HR. Nejati S, et al. Microb Pathog. 2018 Apr;117:43-48. doi: 10.1016/j.micpath.2018.02.016. Epub 2018 Feb 9. Microb Pathog. 2018. PMID: 29432909 Review. - Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis.
Sukri A, Hanafiah A, Mohamad Zin N, Kosai NR. Sukri A, et al. APMIS. 2020 Feb;128(2):150-161. doi: 10.1111/apm.13034. APMIS. 2020. PMID: 32352605 Review. - Pathogenesis of Helicobacter pylori infection.
Maeda S, Mentis AF. Maeda S, et al. Helicobacter. 2007 Oct;12 Suppl 1:10-4. doi: 10.1111/j.1523-5378.2007.00529.x. Helicobacter. 2007. PMID: 17727454 Review.
Cited by
- Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies.
Ali A, AlHussaini KI. Ali A, et al. Microorganisms. 2024 Jan 22;12(1):222. doi: 10.3390/microorganisms12010222. Microorganisms. 2024. PMID: 38276207 Free PMC article. Review. - Changes in the tissue elements of the gastric mucosa interacting with different strains of Helicobacter pylori, taking into consideration the patient's genotype.
Molodozhnikova N, Berestova A, Berechikidze I, Shorina D, Morugina O. Molodozhnikova N, et al. Biosci Microbiota Food Health. 2024;43(3):213-221. doi: 10.12938/bmfh.2023-070. Epub 2024 Mar 19. Biosci Microbiota Food Health. 2024. PMID: 38966050 Free PMC article. - Helicobacter pylori infection impairs chaperone-assisted maturation of Na-K-ATPase in gastric epithelium.
Marcus EA, Tokhtaeva E, Jimenez JL, Wen Y, Naini BV, Heard AN, Kim S, Capri J, Cohn W, Whitelegge JP, Vagin O. Marcus EA, et al. Am J Physiol Gastrointest Liver Physiol. 2020 May 1;318(5):G931-G945. doi: 10.1152/ajpgi.00266.2019. Epub 2020 Mar 16. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32174134 Free PMC article. - Interaction of the microbiota with the human body in health and diseases.
Altveş S, Yildiz HK, Vural HC. Altveş S, et al. Biosci Microbiota Food Health. 2020;39(2):23-32. doi: 10.12938/bmfh.19-023. Epub 2019 Dec 25. Biosci Microbiota Food Health. 2020. PMID: 32328397 Free PMC article. Review. - Helicobacter pylori and oral-gut microbiome: clinical implications.
Elghannam MT, Hassanien MH, Ameen YA, Turky EA, ELattar GM, ELRay AA, ELTalkawy MD. Elghannam MT, et al. Infection. 2024 Apr;52(2):289-300. doi: 10.1007/s15010-023-02115-7. Epub 2023 Nov 2. Infection. 2024. PMID: 37917397 Free PMC article. Review.
References
- Calvet X., Ramírez Lázaro M.J., Lehours P., Mégraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013;18(Suppl. 1):5–11. - PubMed
- Yang X., Li H., Cheng T., Xia W., Lai Y.T., Sun H. Nickel translocation between metallochaperones HypA and UreE in Helicobacter pylori. Metallomics. 2014;6:1731–1736. - PubMed
- Weeks D.L., Eskandari S., Scott D.R., Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485. - PubMed
- Scott D.R., Marcus E.A., Wen Y., Singh S., Feng J., Sachs G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori. J Bacteriol. 2010;192:94–103. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous