Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer - PubMed (original) (raw)
Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer
T Taniguchi et al. Cell Death Dis. 2016.
Abstract
Resveratrol has various attractive bioactivities, such as prevention of cancer, neurodegenerative disorders, and obesity-related diseases. Therefore, identifying its direct binding proteins is expected to discover druggable targets. Sirtuin 1 and phosphodiesterases have so far been found as the direct molecular targets of resveratrol. We herein identified 11 novel resveratrol-binding proteins, including the DEAD (Asp-Glu-Ala-Asp) box helicase 5 (DDX5, also known as p68), using resveratrol-immobilized beads. Treatment with resveratrol induced degradation of DDX5 in prostate cancer cells. Depletion of DDX5 caused apoptosis by inhibiting mammalian target of rapamycin complex 1 (mTORC1) signaling. Moreover, knockdown of DDX5 attenuated the inhibitory activities of resveratrol against mTORC1 signaling and cancer cell growth. These data show that resveratrol directly targets DDX5 and induces cancer cell death by inhibiting the mTORC1 pathway.
Figures
Figure 1
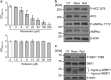
Resveratrol, but not a PDE inhibitor, suppresses the growth of prostate cancer cells. (a) Human prostate cancer PC-3 cells were treated with the indicated concentrations of resveratrol or the PDE4 inhibitor rolipram for 72 h. Relative viability of the cells was measured by CCK-8 assay. Data are means±S.D. (_n_=3). *P<0.05 relative to control (one-way analysis of variance, Bonferroni post-hoc tests). (b and c) Western blotting analysis of PC-3 cells treated with 0.1% DMSO (CT), 100 _μ_M resveratrol (Resv), or 100 _μ_M rolipram (Roli) for 3 h (b) or 24 h (c)
Figure 2
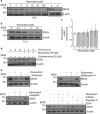
Identification of resveratrol-binding proteins. (a) The scheme for fixation of resveratrol onto magnetic FG beads with epoxy linkers. (b) Purified recombinant PDE4A (2 _μ_g) was incubated with empty (−) or resveratrol-immobilized (+) beads for 4 h, and bound PDE4A was detected by western blotting. The input lane corresponds to recombinant PDE4A (250 ng). (c) Resveratrol-binding proteins were purified from human prostate cancer PC-3 cell extracts, silver-stained, and identified by matrix assisted laser desorption/ionization time-of-flight mass spectrometric analysis. The input lane represents 1% of the PC-3 cell extracts used for the binding assay. (d) In the competitive assay, PC-3 cell extracts were preincubated with the indicated doses of resveratrol for 1 h and incubated with the beads for 15 min. Bound DDX5 was detected by western blotting. The input lane represents 5% of the PC-3 cell extracts used for the binding assay. (e) Purified recombinant DDX5 (1 _μ_g) with or without RNase A was incubated with the beads, and bound DDX5 was detected by western blotting. The input lane corresponds to recombinant DDX5 protein (150 ng)
Figure 3
Treatment with resveratrol degrades DDX5 protein. (a and b) PC-3 cells were treated with the indicated concentrations of resveratrol for 24 h (a) or every 24 h for 72 h (b). DDX5 protein was detected by western blotting. (c) Quantitative reverse transcriptase–PCR analysis of DDX5 mRNA in PC-3 cells treated with resveratrol for 24 h. Data are means±S.D. (_n_=3). (d) PC-3 cells were incubated for the indicated times with or without 50 _μ_M resveratrol and/or 20 _μ_M cycloheximide. DDX5 protein was detected by western blotting. (e) PC-3 cells were incubated for 24 h with or without 20 _μ_M lactacystin, 1 _μ_M bafilomycin A1, 100 _μ_M leupeptin, 100 _μ_M antipain, 1 _μ_M pepstatin A, or 10 mM EDTA and/or 50 _μ_M resveratrol. DDX5 protein was detected by western blotting
Figure 4
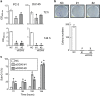
Knockdown of DDX5 inhibits the growth of hormone-independent prostate cancer cells. (a) PC-3 and DU145 cells were transfected with a negative control siRNA (NC), siDDX5 #1, or siDDX5 #2 for 72 or 144 h. Relative viability of the cells was measured by CCK-8 assay. Data are means±S.D. (_n_=3). *P<0.05 relative to control (one-way analysis of variance (ANOVA), Bonferroni post-hoc tests). (b) After PC-3 cells were transfected with or without siDDX5 and incubated for 9 days, colonies were stained with crystal violet and counted (scale bar: 5 mm). Data are means±S.D. (_n_=3). *P<0.05 relative to control (one-way ANOVA, Bonferroni post-hoc tests). (c) PC-3 cells were transfected with or without siDDX5 and incubated for 72, 96, 120, or 144 h, and the percentage of sub-G1 population was quantified by flow cytometry. Data are means±S.D. (_n_=3). *P<0.05 relative to control (one-way ANOVA, Bonferroni post-hoc tests)
Figure 5
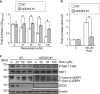
Knockdown of DDX5 inhibits the mTORC1 pathway of hormone-independent prostate cancer cells. (a) Western blotting analysis was performed after PC-3 and DU145 cells were transfected with or without siDDX5 and incubated for 72 h. (b and c) After PC-3 cells were transfected with or without siDDX5 #1 and/or si4EBP1 for 72 h, CCK-8 assay (b) and quantification of apoptosis (c) were performed. Data are means±S.D. (_n_=3). *P<0.05 relative to control (Student's _t_-test)
Figure 6
Resveratrol inhibits the mTORC1 pathway and growth of prostate cancer cells through depletion of DDX5. PC-3 cells were treated with resveratrol at the indicated concentrations for 24 h after transfected with a negative control siRNA (NC) or siDDX5 #1 and incubated for 48 h. (a) Relative viability was measured by CCK-8 assay. Data are means±S.D. (_n_=3). *P<0.05 relative to control (Student's _t_-test). (b) Apoptosis was quantified by flow cytometric analysis. Data are means±S.D. (_n_=3). *P<0.05 relative to control (Student's _t_-test). Resv; Resveratrol. (c) mTORC1 activity was examined by western blotting
Figure 7
A schematic illustration of targeting the DDX5–mTORC1 axis by resveratrol. DDX5 protein is overexpressed in castration-resistant prostate cancer and promotes cell survival and growth by activating mTORC1 signaling. Resveratrol directly binds to DDX5 protein and promotes degradation of DDX5 protein, leading to suppression of the mTORC1 pathway, cancer cell survival, and growth
Similar articles
- Rapamycin attenuates BAFF-extended proliferation and survival via disruption of mTORC1/2 signaling in normal and neoplastic B-lymphoid cells.
Zeng Q, Qin S, Zhang H, Liu B, Qin J, Wang X, Zhang R, Liu C, Dong X, Zhang S, Huang S, Chen L. Zeng Q, et al. J Cell Physiol. 2018 Jan;233(1):516-529. doi: 10.1002/jcp.25913. Epub 2017 May 3. J Cell Physiol. 2018. PMID: 28300280 Free PMC article. - Cucurbitacin E Induces Autophagy via Downregulating mTORC1 Signaling and Upregulating AMPK Activity.
Zha QB, Zhang XY, Lin QR, Xu LH, Zhao GX, Pan H, Zhou D, Ouyang DY, Liu ZH, He XH. Zha QB, et al. PLoS One. 2015 May 13;10(5):e0124355. doi: 10.1371/journal.pone.0124355. eCollection 2015. PLoS One. 2015. PMID: 25970614 Free PMC article. - DDX5 promotes gastric cancer cell proliferation in vitro and in vivo through mTOR signaling pathway.
Du C, Li DQ, Li N, Chen L, Li SS, Yang Y, Hou MX, Xie MJ, Zheng ZD. Du C, et al. Sci Rep. 2017 Feb 20;7:42876. doi: 10.1038/srep42876. Sci Rep. 2017. PMID: 28216662 Free PMC article. - Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation.
Alayev A, Berger SM, Holz MK. Alayev A, et al. Ann N Y Acad Sci. 2015 Aug;1348(1):116-23. doi: 10.1111/nyas.12829. Epub 2015 Jul 22. Ann N Y Acad Sci. 2015. PMID: 26200935 Review. - The role of DEAD-box RNA helicase p68 (DDX5) in the development and treatment of breast cancer.
Hashemi V, Masjedi A, Hazhir-Karzar B, Tanomand A, Shotorbani SS, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Anvari E, Baradaran B, Jadidi-Niaragh F. Hashemi V, et al. J Cell Physiol. 2019 May;234(5):5478-5487. doi: 10.1002/jcp.26912. Epub 2018 Nov 11. J Cell Physiol. 2019. PMID: 30417346 Review.
Cited by
- The Fra-1/AP-1 Oncoprotein: From the "Undruggable" Transcription Factor to Therapeutic Targeting.
Casalino L, Talotta F, Cimmino A, Verde P. Casalino L, et al. Cancers (Basel). 2022 Mar 14;14(6):1480. doi: 10.3390/cancers14061480. Cancers (Basel). 2022. PMID: 35326630 Free PMC article. Review. - DDX5 and DDX17-multifaceted proteins in the regulation of tumorigenesis and tumor progression.
Xu K, Sun S, Yan M, Cui J, Yang Y, Li W, Huang X, Dou L, Chen B, Tang W, Lan M, Li J, Shen T. Xu K, et al. Front Oncol. 2022 Aug 3;12:943032. doi: 10.3389/fonc.2022.943032. eCollection 2022. Front Oncol. 2022. PMID: 35992805 Free PMC article. Review. - DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer.
Le TK, Cherif C, Omabe K, Paris C, Lannes F, Audebert S, Baudelet E, Hamimed M, Barbolosi D, Finetti P, Bastide C, Fazli L, Gleave M, Bertucci F, Taïeb D, Rocchi P. Le TK, et al. Mol Ther. 2023 Feb 1;31(2):471-486. doi: 10.1016/j.ymthe.2022.08.005. Epub 2022 Aug 13. Mol Ther. 2023. PMID: 35965411 Free PMC article. - The DDX5/Dbp2 subfamily of DEAD-box RNA helicases.
Xing Z, Ma WK, Tran EJ. Xing Z, et al. Wiley Interdiscip Rev RNA. 2019 Mar;10(2):e1519. doi: 10.1002/wrna.1519. Epub 2018 Dec 2. Wiley Interdiscip Rev RNA. 2019. PMID: 30506978 Free PMC article. Review. - Genomic sequencing, genome-scale metabolic network reconstruction, and in silico flux analysis of the grape endophytic fungus Alternaria sp. MG1.
Lu Y, Ye C, Che J, Xu X, Shao D, Jiang C, Liu Y, Shi J. Lu Y, et al. Microb Cell Fact. 2019 Jan 24;18(1):13. doi: 10.1186/s12934-019-1063-7. Microb Cell Fact. 2019. PMID: 30678677 Free PMC article.
References
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–220. - PubMed
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5: 493–506. - PubMed
- Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 2009; 119: 1643–1652. - PubMed
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992; 339: 1523–1526. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical