Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice - PubMed (original) (raw)
Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice
Kong-Yan Wu et al. Cell Res. 2016 Jun.
Abstract
The recent Zika virus (ZIKV) epidemic in Latin America coincided with a marked increase in microcephaly in newborns. However, the causal link between maternal ZIKV infection and malformation of the fetal brain has not been firmly established. Here we show a vertical transmission of ZIKV in mice and a marked effect on fetal brain development. We found that intraperitoneal (i.p.) injection of a contemporary ZIKV strain in pregnant mice led to the infection of radial glia cells (RGs) of dorsal ventricular zone of the fetuses, the primary neural progenitors responsible for cortex development, and caused a marked reduction of these cortex founder cells in the fetuses. Interestingly, the infected fetal mice exhibited a reduced cavity of lateral ventricles and a discernable decrease in surface areas of the cortex. This study thus supports the conclusion that vertically transmitted ZIKV affects fetal brain development and provides a valuable animal model for the evaluation of potential therapeutic or preventative strategies.
Figures
Figure 1
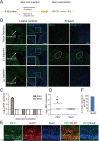
Vertical transmission of ZIKV and targeting of radial glia cells in fetal mice. (A) Experimental procedures of ZIKV infection in mouse. ZIKV was intraperitoneally (i.p.) injected into pregnant C57 mice at E13.5 or directly injected into the lateral ventricle of the fetus, followed by brain examination on E17.5 or P1. (B) P1 mouse brain slices were stained with convalescent phase serum and DAPI which labels the nucleus. Shown are representative images from 5 mice. Note the virus signals in VZ regions in ZIKV-injected group and nonspecific background signal in mock i.p. injected group. Scale bar, 100 μm. (C) Viremia of ZIKV-infected mice by i.p. route. Pregnant mice were inoculated with ZIKV, and viremia was determined on 1, 2 and 3 days post inoculation by real-time RT-PCR. Dotted lines represent limits of detection. (D) Placenta of each embryo was collected at 3 days post inoculation and viral titers was determined by real-time RT-PCR. Dotted line represents limits of detection. (E) Immunostaining for radial glia marker BLBP and stem cell marker Sox2 in brain slices from P1 mice i.p injected with ZIKV at E13.5. Arrows indicate ZIKV and BLBP colocalized signals. Scale bar, 50 μm. (F) Quantification of the percentage of ZIKV signals colocalized with BLBP. Data are the average of five mouse brains.
Figure 2
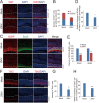
ZIKV inhibits neural stem cell proliferation and depletes progenitor pool in dorsal ventricular zone. (A) Immunostaining for Ki67 in brain slices from P1 mice i.p. injected with ZIKV or mock control at E13.5. White dash lines indicate the boundary between VZ/SVZ and IZ zones. Scale bar, 50 μm. (B) Quantification for the number of Ki67-positive (Ki67+) cells in VZ/SVZ and IZ regions from mock and ZIKV groups. Data are shown as mean ± SEM (*P < 0.05, **P< 0.01; Student's _t_-test). (C) Immunostaining for BLBP and Sox2 in brain slices from P1 mice i.p. injected with ZIKV or mock control at E13.5. White dash lines indicate the boundary between VZ/SVZ and IZ zones. Yellow dash lines indicate the densely arrayed continuous Sox2-positive (Sox2+) cell layers within the VZ/SVZ region. Scale bars, 50 μm. (D, E) Quantification for the band thickness of densely arrayed Sox2+ cell layers relative to that of the entire VZ/SVZ region (D) and the intensity of Sox2+ or BLBP+ cells (E). Data are mean ± SEM (*P< 0.05, **P< 0.01, ***P< 0.001, Student's _t_-test). (F) Immunostaining for Tbr2 in brain slices from P1 mice i.p. injected with ZIKV or mock control at E13.5. White dash lines indicate boundary between VZ/SVZ and IZ zones. Scale bars, 50 μm. (G, H) Quantification of relative intensity of Tbr2+ cells (G) and the band thickness of densely arrayed Tbr2+ cell layers relative to that of the entire VZ/SVZ region (H). Data are mean ± SEM (**P< 0.05, ***P< 0.001, Student's _t_-test).
Figure 3
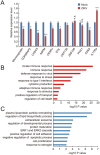
Effects of ZIKV infection on gene expression. (A) Quantitative real-time PCR analysis of the mRNA levels of cell cycle-related and microcephaly-related genes in ZIKV-injected E17.5 fetal brains. GAPDH was used as the internal control. Data are mean ± SEM from 3 experiments (*P< 0.05, **P< 0.01, ***P< 0.001, NS, no significant difference, Student's _t_-test). (B, C) RNA-seq and GO analyses reveal pathways that are significantly up-regulated (B) and significantly down-regulated (C) in ZIKV-injected E17.5 fetal brains compared with control samples. Bar plots show the –log10 P values of each term of genes.
Figure 4
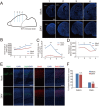
Effect of ZIKV infection on brain development of fetal mice. (A) Serial coronal brain sections as illustrated (left) were stained with DAPI (right). Numbers indicate selected corresponding sections. Scale bar, 500 μm. (B-D) Quantification of the length of outer cortical surface (B), the lateral ventricle area (C), and the perimeter lining the ventricular surface (D) in indicated sections. Data are shown as mean ± SEM from three offspring mice (*P< 0.05, **P< 0.01, ***P< 0.001, NS, no significant difference, Student's _t_-test). (E) E17.5 brain slices in corresponding position from mock or ZIKV-infected group were stained with antibodies against superfical layer marker Satb2 or deep layer marker Ctip2, respectively. Scale bar, 100 μm. (F) Quantification of the relative thickness of Satb2 or Ctip2 positive layers relative to the total cortex expressed as a percentage with the thickness of the entire cortex being 100%. There is no significant difference between two groups in each layer.
Comment in
- Neural stem cells attacked by Zika virus.
Nguyen HN, Qian X, Song H, Ming GL. Nguyen HN, et al. Cell Res. 2016 Jul;26(7):753-4. doi: 10.1038/cr.2016.68. Epub 2016 Jun 10. Cell Res. 2016. PMID: 27283801 Free PMC article.
Similar articles
- Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon.
Gurung S, Reuter N, Preno A, Dubaut J, Nadeau H, Hyatt K, Singleton K, Martin A, Parks WT, Papin JF, Myers DA. Gurung S, et al. PLoS Pathog. 2019 Jan 18;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30657788 Free PMC article. - Role of microglia in the dissemination of Zika virus from mother to fetal brain.
Xu P, Shan C, Dunn TJ, Xie X, Xia H, Gao J, Allende Labastida J, Zou J, Villarreal PP, Schlagal CR, Yu Y, Vargas G, Rossi SL, Vasilakis N, Shi PY, Weaver SC, Wu P. Xu P, et al. PLoS Negl Trop Dis. 2020 Jul 6;14(7):e0008413. doi: 10.1371/journal.pntd.0008413. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32628667 Free PMC article. - Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know.
Alvarado MG, Schwartz DA. Alvarado MG, et al. Arch Pathol Lab Med. 2017 Jan;141(1):26-32. doi: 10.5858/arpa.2016-0382-RA. Epub 2016 Sep 16. Arch Pathol Lab Med. 2017. PMID: 27636525 Review. - Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice.
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Li C, et al. Cell Stem Cell. 2016 Jul 7;19(1):120-6. doi: 10.1016/j.stem.2016.04.017. Epub 2016 May 11. Cell Stem Cell. 2016. PMID: 27179424 - Zika virus infection and pregnancy: what we do and do not know.
Ticconi C, Pietropolli A, Rezza G. Ticconi C, et al. Pathog Glob Health. 2016 Oct-Dec;110(7-8):262-268. doi: 10.1080/20477724.2016.1234804. Epub 2016 Sep 30. Pathog Glob Health. 2016. PMID: 27690200 Free PMC article. Review.
Cited by
- Navigating the Zika panic.
Grubaugh ND, Andersen KG. Grubaugh ND, et al. F1000Res. 2016 Aug 4;5:1914. doi: 10.12688/f1000research.9370.1. eCollection 2016. F1000Res. 2016. PMID: 27746903 Free PMC article. - Human astrocytes are distinct contributors to the complexity of synaptic function.
Krencik R, van Asperen JV, Ullian EM. Krencik R, et al. Brain Res Bull. 2017 Mar;129:66-73. doi: 10.1016/j.brainresbull.2016.08.012. Epub 2016 Aug 25. Brain Res Bull. 2017. PMID: 27570101 Free PMC article. Review. - Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication.
Hammack C, Ogden SC, Madden JC Jr, Medina A, Xu C, Phillips E, Son Y, Cone A, Giovinazzi S, Didier RA, Gilbert DM, Song H, Ming G, Wen Z, Brinton MA, Gunjan A, Tang H. Hammack C, et al. J Virol. 2019 Sep 30;93(20):e00638-19. doi: 10.1128/JVI.00638-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375586 Free PMC article. - Zika Virus Neuropathogenesis: The Different Brain Cells, Host Factors and Mechanisms Involved.
Komarasamy TV, Adnan NAA, James W, Balasubramaniam VRMT. Komarasamy TV, et al. Front Immunol. 2022 Mar 16;13:773191. doi: 10.3389/fimmu.2022.773191. eCollection 2022. Front Immunol. 2022. PMID: 35371036 Free PMC article. Review. - A gossypol derivative effectively protects against Zika and dengue virus infection without toxicity.
Gao Y, Tai W, Wang X, Jiang S, Debnath AK, Du L, Chen S. Gao Y, et al. BMC Biol. 2022 Jun 15;20(1):143. doi: 10.1186/s12915-022-01344-w. BMC Biol. 2022. PMID: 35706035 Free PMC article.
References
- Oehler E, Fournier E, Leparc-Goffart I, et al. Increase in cases of Guillain-Barre syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveill 2015; 20:30079. - PubMed
- Ioos S, Mallet HP, Leparc Goffart I, et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307. - PubMed
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7. - PubMed
- Fauci AS, Morens DM. Zika Virus in the Americas--yet another arbovirus threat. N Engl J Med 2016; 374:601–604. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical