Direct Comparison of Manganese Detoxification/Efflux Proteins and Molecular Characterization of ZnT10 Protein as a Manganese Transporter - PubMed (original) (raw)
Comparative Study
. 2016 Jul 8;291(28):14773-87.
doi: 10.1074/jbc.M116.728014. Epub 2016 May 10.
Natsuko Tsuji 1, Hitomi Fujishiro 2, Taka-Aki Takeda 1, Tomohiro Yamazaki 1, Fumie Teranishi 1, Fumiko Okazaki 3, Ayu Matsunaga 3, Karin Tuschl 4, Rajini Rao 5, Satoshi Kono 6, Hiroaki Miyajima 6, Hiroshi Narita 3, Seiichiro Himeno 2, Taiho Kambe 7
Affiliations
- PMID: 27226609
- PMCID: PMC4938194
- DOI: 10.1074/jbc.M116.728014
Comparative Study
Direct Comparison of Manganese Detoxification/Efflux Proteins and Molecular Characterization of ZnT10 Protein as a Manganese Transporter
Yukina Nishito et al. J Biol Chem. 2016.
Abstract
Manganese homeostasis involves coordinated regulation of specific proteins involved in manganese influx and efflux. However, the proteins that are involved in detoxification/efflux have not been completely resolved nor has the basis by which they select their metal substrate. Here, we compared six proteins, which were reported to be involved in manganese detoxification/efflux, by evaluating their ability to reduce manganese toxicity in chicken DT40 cells, finding that human ZnT10 (hZnT10) was the most significant contributor. A domain swapping and substitution analysis between hZnT10 and the zinc-specific transporter hZnT1 showed that residue Asn(43), which corresponds to the His residue constituting the potential intramembranous zinc coordination site in other ZnT transporters, is necessary to impart hZnT10's unique manganese mobilization activity; residues Cys(52) and Leu(242) in transmembrane domains II and V play a subtler role in controlling the metal specificity of hZnT10. Interestingly, the His → Asn reversion mutant in hZnT1 conferred manganese transport activity and loss of zinc transport activity. These results provide important information about manganese detoxification/efflux mechanisms in vertebrate cells as well as the molecular characterization of hZnT10 as a manganese transporter.
Keywords: ATP13A family protein; SPCA1; efflux; ferroportin; manganese; metal homeostasis; substrate specificity; transporter; zinc.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
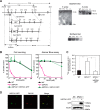
_SPCA1_−/−/− cells show significantly reduced resistance to high manganese concentrations. A, targeted disruption of the c_SPCA1_ gene. Three targeting constructs were designed to disrupt the exon encoding actuator domain. The HisD, Bsr, or Puro drug-resistant marker cassettes were flanked by mutated loxP sites indicated by gray arrowheads. Gray boxes indicate the position of 5′ and 3′ probes. Southern blotting analyses (right upper panels) and Northern blotting analyses (right bottom panel) confirmed the disruption of the SPCA1 gene. B, _SPCA1_−/−/− cells were significantly sensitive to high manganese concentrations. Cells were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the number of living cells was counted (left graph) and evaluated by the Alamar Blue assay (right graph). Relative values are plotted as a percentage of living cells without MnSO4 for each group of cells. The growth curves of wild-type (WT), _SPCA1_−/−/−, and _SPCA1_−/−/− stably expressing hSPCA1-GFP are shown. Each experiment was performed at least three times. Note that hSPCA1-GFP expression reversed the phenotypes of _SPCA1_−/−/− cells. C, _SPCA1_−/−/− cells accumulated high manganese concentrations in the cells. Amounts of manganese in the cells were evaluated by measuring 54Mn accumulated in the cells cultured for 24 h in the presence of 10 μ
m
54MnCl2. Each value is the mean ± S.D. of three independent experiments (*, p < 0.01). Note that hSPCA1-GFP expression decreased the accumulation of 54Mn in _SPCA1_−/−/− cells, although the level of accumulated 54Mn in the cells at 24 h does not necessarily reflect steady state levels of cellular manganese. D, subcellular localization of hSPCA1 expressed in _SPCA1_−/−/− cells. hSPCA1-GFP (green), GM130 (red), and the merged images are shown. Confirmation of stable hSPCA1-GFP expression in _SPCA1_−/−/− cells by immunoblotting is shown. Ten micrograms of total cellular protein was loaded onto each lane, and the same membrane was used for detection of both hSPCA1 and tubulin. Tubulin is shown as a loading control.
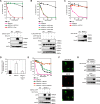
FIGURE 2.
Evaluation of the conferment of manganese detoxification using _SPCA1_−/−/− cells. A, hFpn failed to reverse the phenotypes of _SPCA1_−/−/− cells. The growth curves of wild-type (WT), _SPCA1_−/−/−, and _SPCA1_−/−/− stably expressing hSPCA1-GFP and _SPCA1_−/−/− stably expressing hFpn-V5 are shown. Confirmation of stable hFpn and hSPCA1 expression in _SPCA1_−/−/− cells by immunoblotting (lower panels) is shown. B, hATP13A1, hATP13A2, and hATP13A3 had almost no effect on manganese resistance in _SPCA1_−/−/− cells. The growth curves of wild-type (WT), _SPCA1_−/−/−, and _SPCA1_−/−/− stably expressing FLAG-hATP13A1, hATP13A2-HA, or HA-hATP13A3 are shown. Confirmation of stable expression of hATP13A1, hATP13A2, and hATP13A3 in _SPCA1_−/−/− cells by immunoblotting (lower panels) is shown. C, hZnT10 completely reversed the phenotypes of _SPCA1_−/−/− cells. The growth curves of wild-type (WT), _SPCA1_−/−/−, and _SPCA1_−/−/− stably expressing hZnT10-HA are shown. Confirmation of stable hZnT10 expression in _SPCA1_−/−/− cells by immunoblotting (lower panels) is shown. D, hZnT10 decreased accumulated 54Mn in SPCA1_−/−/− cells. Amounts of accumulated 54Mn in the cells at 24 h were evaluated as in Fig. 1_C. Each value is the mean ± S.D. of three independent experiments (*, p < 0.01). E, co-expression of hZnT10 with hSPCA1 conferred more resistance to high manganese concentrations than that of single expression of hSPCA1. The growth curves of wild-type (WT), _SPCA1_−/−/−, and _SPCA1_−/−/− stably expressing hZnT10, _SPCA1_−/−/− stably expressing hSPCA1, and _SPCA1_−/−/−− stably expressing both hZnT10 and hSPCA1 are shown. Confirmation of stable hZnT10 and hSPCA1 expression in _SPCA1_−/−/− cells by immunoblotting (lower panels) is shown. Note that co-expression of hZnT10 with hSPCA1 conferred greater resistance to high manganese concentrations compared with that of only hSPCA1 expression. F, immunofluorescence staining of hZnT10 expressed in _SPCA1_−/−/− cells. hZnT10 (green), GM130 (red), and the merged images are shown. G, cell surface localization of hZnT10 evaluated by the surface biotinylation assay. Cells treated with the biotinylation reagent (sulfo-NHS-SS-biotin) were solubilized, and the biotinylated protein was then captured using streptavidin beads and analyzed by immunoblot analysis. Input refers to aliquots of the biotinylated proteins before avidin capture (i.e. total cell lysate), although biotinylation refers to avidin-captured proteins. Tubulin and IgM were used as loading controls for input and biotinylation, respectively. The representative results of three independent experiments are displayed. A–C and E, cells were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the numbers of living cells were evaluated by the Alamar Blue assay at least three times. Tubulin and calnexin (CNX) are shown as the loading controls.
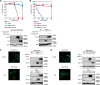
FIGURE 3.
ZnT10 is involved in manganese transport rather than zinc transport. A, expression of hZnT10 did not restore zinc resistance of _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. Cells stably expressing hZnT10 or hZnT1 were grown in the presence of the indicated concentrations of ZnSO4 for 2 days, and the numbers of living cells were evaluated by the Alamar Blue assay. Confirmation of stable hZnT10 or hZnT1 expression in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. B, expression of hZnT1 failed to confer manganese resistance of SPCA1_−/−/− cells. Cells stably expressing hZnT1 or hZnT10 were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the number of living cells were evaluated by the Alamar Blue assay. Confirmation of stable hZnT10 or hZnT1 expression in SPCA1_−/−/− cells by immunoblotting (lower panels) is shown. A and B, Alamar Blue assay was performed at least three times. Calnexin (CNX) is shown as the loading controls. C, plasma membrane localizations of hZnT1 and hZnT10 expressed in SPCA1_−/−/−cells is shown. Immunofluorescence staining of both proteins was performed as presented in Fig. 2_F). The biotinylation assay was performed as in Fig. 2_G. D, plasma membrane localizations of hZnT1 and hZnT10 expressed in ZnT1_−/−_MT_−/−_ZnT4_−/− cells are shown. Immunofluorescence staining of both proteins was performed as in Fig. 2_F. The biotinylation assay was performed as in Fig. 2_G. C and D, tubulin and IgM were used as loading controls for input and biotinylation, respectively. The representative results of three independent experiments are presented.
FIGURE 4.
Multiple sequence alignment of TMDs II and V among hZnT transporters. The sequences of TMDs II and V of hZnT transporters were aligned. The sequence order is according to their sequence similarity (41). The conserved His and Asp residues postulated as the zinc-binding site in TMDs II and V are highlighted in orange and blue. Residue Asn43 in TMD II of hZnT10 is highlighted in green. Cys52 and Leu242 residues of hZnT10 that were investigated in Fig. 9 are shown in red. The indicated TMDs of hZnT5 correspond to TMDs XI and XIV.
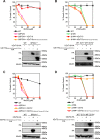
FIGURE 5.
Asn residue in TMD II of hZnT10 is essential for manganese transport activity. A, expression of hZnT10(hZnT1Cter) did not alter manganese resistance in _SPCA1_−/−/− cells. B, expression of hZnT10(hZnT1Cter) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. C, expression of hZnT10(hZnT1Loop) did not alter manganese resistance in _SPCA1_−/−/− cells. D, expression of hZnT10(hZnT1Loop) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. E, expression of hZnT10(N43H) significantly decreased manganese resistance in _SPCA1_−/−/− cells. F, expression of hZnT10(N43H) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. In A, C, and E or in B, D, and F, cells were grown as in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. In A–F, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT10 in _SPCA1_−/−/− cells or _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as a loading control. G, cell surface localization of hZnT10 mutants in _SPCA1_−/−/− cells was evaluated by the surface biotinylation assay. H, the cell surface localization of hZnT10 mutants in ZnT1_−/−_MT_−/−_ZnT4_−/− cells was evaluated by the surface biotinylation assay. In G and H, the biotinylation assay was performed as in Fig. 2_G. The representative results of three independent experiments are presented.
FIGURE 6.
Substitution of Asn residue for His residue in TMD II confers the activity to transport manganese with hZnT1. A, expression of hZnT1(hZnT10Cter) did not confer manganese resistance in _SPCA1_−/−/− cells. B, expression of hZnT1(hZnT10Cter) did not alter zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. C, expression of hZnT1(hZnT10Loop) did not confer manganese resistance in _SPCA1_−/−/− cells. D, expression of hZnT1(hZnT10Loop) did not alter zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. E, expression of hZnT1(H43N) did confer manganese resistance in _SPCA1_−/−/− cells. F, expression of hZnT1(H43N) lost the ability to confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. In A, C, and E or in B, D, and F, cells were grown as in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–F, Alamar Blue assay was performed at least three times. Confirmation of stable WT and mutants of hZnT1 expression in _SPCA1_−/−/− cells or _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. Tubulin and calnexin (CNX) are shown as the loading controls. G, cell surface localization of hZnT1 mutants in _SPCA1_−/−/− cells was evaluated by the surface biotinylation assay. H, cell surface localization of hZnT1 mutants in ZnT1_−/−_MT_−/−_ZnT4_−/− cells was evaluated by the surface biotinylation assay. G and H, hZnT1(hZnT10Cter) was detected by an anti-hZnT10 antibody, whereas WT ZnT1 and other hZnT1 mutants were detected by an anti-hZnT1 antibody. The biotinylation assay was performed as in Fig. 2_G. The representative results of three independent experiments are displayed.
FIGURE 7.
Domain swapping and substitution analysis between hZnT10 and hZnT2. A, expression of hZnT2(H106N) failed to confer manganese resistance in _SPCA1_−/−/− cells. B, expression of hZnT2(H106N) lost the ability to confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. C, expression of hZnT10(hZnT2Cter) lost the ability to confer manganese resistance in _SPCA1_−/−/− cells. D, expression of hZnT10(hZnT2Cter) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. E, expression of hZnT2(hZnT10Cter) did not confer manganese resistance in _SPCA1_−/−/− cells. F, expression of hZnT2(hZnT10Cter) lost the ability to confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. In A, C, and E or in B, D, and F, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–F, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT2 and hZnT10 in _SPCA1_−/−/− cells or _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
FIGURE 8.
Domain swapping and substitution of specific sequences failed to be compatible between hZnT10 and hZnT2. A, expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) lost the ability to confer manganese resistance in _SPCA1_−/−/− cells. B, expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. C, expression of hZnT2(H106N-hZnT10Loop-hZnT10Cter) did not confer manganese resistance in _SPCA1_−/−/− cells. D, expression of hZnT2(H106N-hZnT10Loop-hZnT10Cter) lost the ability to zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. In A and C or in B and D, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–D, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT2 and hZnT10 in _SPCA1_−/−/−cells or _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
FIGURE 9.
Residues Cys52 and Leu242 in the TMDs II and IV of hZnT10 are involved in the control of metal substrate specificity in hZnT10. A, expression of hZnT10(N43H,C52V) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. B, expression of hZnT10(N43H,L242F) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. C, expression of hZnT10(N43H,C52V,L242F) and hZnT10(N43H,C52V,L242F-hZnT1Cter) partially restored the ability to confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. D, expression of hZnT10(N43H-hZnT1Cter) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. E, expression of hZnT10(C52V,L242F) did not confer zinc resistance in _ZnT1_−/−_MT_−/−_ZnT4_−/− cells. F, expression of hZnT10(C52V,L242F) did not impair manganese resistance in _SPCA1_−/−/− cells. In A–E or in F, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. The Alamar Blue assay was performed at least three times in A and B and D–F and four times in C. Confirmation of stable expression of hZnT10 mutants in _SPCA1_−/−/− cells or _ZnT1_−/−_MT_−/−_ZnT4_−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
Similar articles
- Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters.
Zogzas CE, Mukhopadhyay S. Zogzas CE, et al. Metallomics. 2018 Aug 15;10(8):1053-1064. doi: 10.1039/c8mt00115d. Metallomics. 2018. PMID: 29989630 Free PMC article. - Structural Elements in the Transmembrane and Cytoplasmic Domains of the Metal Transporter SLC30A10 Are Required for Its Manganese Efflux Activity.
Zogzas CE, Aschner M, Mukhopadhyay S. Zogzas CE, et al. J Biol Chem. 2016 Jul 29;291(31):15940-57. doi: 10.1074/jbc.M116.726935. Epub 2016 Jun 15. J Biol Chem. 2016. PMID: 27307044 Free PMC article. - Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn2+ is driven by an active Ca2+-coupled exchange.
Levy M, Elkoshi N, Barber-Zucker S, Hoch E, Zarivach R, Hershfinkel M, Sekler I. Levy M, et al. J Biol Chem. 2019 Apr 12;294(15):5879-5889. doi: 10.1074/jbc.RA118.006816. Epub 2019 Feb 12. J Biol Chem. 2019. PMID: 30755481 Free PMC article. - Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP.
Fujishiro H, Kambe T. Fujishiro H, et al. J Pharmacol Sci. 2022 Jan;148(1):125-133. doi: 10.1016/j.jphs.2021.10.011. Epub 2021 Nov 2. J Pharmacol Sci. 2022. PMID: 34924116 Review. - Familial manganese-induced neurotoxicity due to mutations in SLC30A10 or SLC39A14.
Mukhopadhyay S. Mukhopadhyay S. Neurotoxicology. 2018 Jan;64:278-283. doi: 10.1016/j.neuro.2017.07.030. Epub 2017 Aug 5. Neurotoxicology. 2018. PMID: 28789954 Free PMC article. Review.
Cited by
- Bile acid composition regulates the manganese transporter Slc30a10 in intestine.
Ahmad TR, Higuchi S, Bertaggia E, Hung A, Shanmugarajah N, Guilz NC, Gamarra JR, Haeusler RA. Ahmad TR, et al. J Biol Chem. 2020 Aug 28;295(35):12545-12558. doi: 10.1074/jbc.RA120.012792. Epub 2020 Jul 20. J Biol Chem. 2020. PMID: 32690612 Free PMC article. - Implications for Cation Selectivity and Evolution by a Novel Cation Diffusion Facilitator Family Member From the Moderate Halophile Planococcus dechangensis.
Xu T, Chen H, Li J, Hong S, Shao L, Zheng X, Zou Q, Wang Y, Guo S, Jiang J. Xu T, et al. Front Microbiol. 2019 Mar 22;10:607. doi: 10.3389/fmicb.2019.00607. eCollection 2019. Front Microbiol. 2019. PMID: 30967858 Free PMC article. - Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters.
Zogzas CE, Mukhopadhyay S. Zogzas CE, et al. Metallomics. 2018 Aug 15;10(8):1053-1064. doi: 10.1039/c8mt00115d. Metallomics. 2018. PMID: 29989630 Free PMC article. - Metal transport mechanism of the cation diffusion facilitator (CDF) protein family - a structural perspective on human CDF (ZnT)-related diseases.
Barber-Zucker S, Moran A, Zarivach R. Barber-Zucker S, et al. RSC Chem Biol. 2021 Jan 25;2(2):486-498. doi: 10.1039/d0cb00181c. eCollection 2021 Apr 1. RSC Chem Biol. 2021. PMID: 34458794 Free PMC article. Review. - Metal Transporter Zip14 (Slc39a14) Deletion in Mice Increases Manganese Deposition and Produces Neurotoxic Signatures and Diminished Motor Activity.
Aydemir TB, Kim MH, Kim J, Colon-Perez LM, Banan G, Mareci TH, Febo M, Cousins RJ. Aydemir TB, et al. J Neurosci. 2017 Jun 21;37(25):5996-6006. doi: 10.1523/JNEUROSCI.0285-17.2017. Epub 2017 May 23. J Neurosci. 2017. PMID: 28536273 Free PMC article.
References
- Wood R. J. (2009) Manganese and birth outcome. Nutr. Rev. 67, 416–420 - PubMed
- Lucchini R. G., Martin C. J., and Doney B. C. (2009) From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med. 11, 311–321 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous