Structural insights of ZIP4 extracellular domain critical for optimal zinc transport - PubMed (original) (raw)
Structural insights of ZIP4 extracellular domain critical for optimal zinc transport
Tuo Zhang et al. Nat Commun. 2016.
Abstract
The ZIP zinc transporter family is responsible for zinc uptake from the extracellular milieu or intracellular vesicles. The LIV-1 subfamily, containing nine out of the 14 human ZIP proteins, is featured with a large extracellular domain (ECD). The critical role of the ECD is manifested by disease-causing mutations on ZIP4, a representative LIV-1 protein. Here we report the first crystal structure of a mammalian ZIP4-ECD, which reveals two structurally independent subdomains and an unprecedented dimer centred at the signature PAL motif. Structure-guided mutagenesis, cell-based zinc uptake assays and mapping of the disease-causing mutations indicate that the two subdomains play pivotal but distinct roles and that the bridging region connecting them is particularly important for ZIP4 function. These findings lead to working hypotheses on how ZIP4-ECD exerts critical functions in zinc transport. The conserved dimeric architecture in ZIP4-ECD is also demonstrated to be a common structural feature among the LIV-1 proteins.
Figures
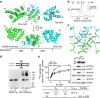
Figure 1. The role of ZIP4-ECD in ZIP4-mediated zinc transport.
(a) Cell-based zinc uptake assay. HEK293T cells transiently expressing hZIP4 and hZIP4-ΔECD were incubated with the indicated concentrations of ZnCl2 (containing 20% 65ZnCl2) in zinc uptake buffer at 37 °C for 20 min. After rapid washing on ice, the cells were lysed and the amount of zinc was measured using a Gamma counter. The cells transfected with the empty vector were used as control. The raw experimental data (left) and the processed data (right) after subtracting the zinc uptake in cells transfected with empty vector represent the results of one out of three independent experiments. The data from the other two experiments are shown in Supplementary Fig. 1 in Supplementary Information. Each data point represents the mean of three replicates. The error bars indicate 1±s.d. The results of curve fitting using the Hill model are shown in the Table 1 (c). (b) top: western blot of hZIP4 and hZIP4-ΔECD expressed in HEK293T cells using anti-HA antibody. The glycosylated (upper band) and un-glycosylated (lower band) proteins are indicated by arrows. bottom: comparison of the surface expression levels of hZIP4 and hZIP4-ΔECD with three replicates. The surface expression level is indicated by the surface bound anti-HA antibody. β-actin was detected using anti-β-actin antibody in western blot as loading control. (c) Zinc transport kinetic parameters of hZIP4 and hZIP4-ΔECD. The units of _K_m and _V_max are μM and pmol min−1 mg−1, respectively. To obtain the calibrated _V_max (_V_max, c), the apparent _V_max (Vmax, a) calculated from curve fitting (OriginPro 8.5) was divided by the averaged surface expression level determined by Image Lab (Bio Rad) programme. The surface expression level of the corresponding protein is expressed as a ratio relative to that of the wild-type hZIP4. The calibrated _V_maxs were then normalized by dividing the calibrated _V_max of the wild-type hZIP4 and expressed as percentages. (d) Statistical analysis of the normalized _V_maxs (_V_max, n) from three independent experiments. The Student's _t_-test is used to assess significance statistically.***P<0.001.
Figure 2. Crystal structure of pZIP4-ECD.
(a) The structure of pZIP4-ECD dimer. The helices are presented as cylinders. Only the helices in the green protomer are labelled, except that α12 in the blue protomer is labelled. It is because α12 in the green molecule is seriously disordered. The disordered sequences are shown as dashed lines. Key structural elements, including the H-P linker, L12–13 and the histidine-rich loop, are indicted by arrows. (b) Multiple sequence alignment of mammalian ZIP4-ECDs. The Genebank codes and NCBI reference sequence codes are: AAH62625 for human, ELK11751 for bat, XP_004580947 for rabbit, XP_003760599 for Sarcophilus and XP_001371869 for Monodelphis. The predicted signal peptides are shown in grey, the HRD in orange and the PCD in purple. The invariant cysteine residues are highlighted in yellow and the disulfide bonds are indicated with blue lines. The residues subjected to mutations in AE are highlighted in black blocks. The residues involved in dimerization are labelled with black triangles. The secondary structures derived from pZIP4-ECD structure are shown above the sequences. The dashed lines indicate the disordered regions.
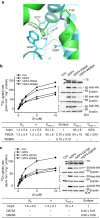
Figure 3. Dimerization of pZIP4-ECD.
(a) Top view and side view of pZIP4-ECD. The PAL motif at the centre of the dimerization interface is indicated in the top view. The putative membrane surface and the TMD are illustrated in the side view. The disordered histidine rich loop and the linker connecting the ECD and the TMD are depicted as dashed lines. (b) Size exclusion chromatography profiles of pZIP4-ECD and a truncated construct terminated at the end of α11. The peaks of the standard proteins eluted on the same column are indicated by arrows labelled with the molecular weights. (c) The zoomed-in structure of the PAL motif in the top view. The labelled residues in 293SPALL297 sequence are shown as stick. The other conserved residues around the PAL motif are shown in line model. (d) Western blots of the wild-type hZIP4 and the S297C mutant in the presence (left) and absence (right) of 5% β-mercaptoethanol. As shown in the sequence alignment, S297 in hZIP4 is equivalent to S293 in pZIP4. A 4–20% gradient SDS–PAGE gel was used for better resolution at high molecular weight region. (e) Functional characterization of hZIP4 P298A and L300A double mutant (PL). left: zinc uptake assay. The shown data are the results of one out of three independent experiments. The data from the other two experiments are shown in Supplementary Fig. 8a,b. Each data point represents the mean of three replicates. The error bars indicate 1±s.d. right: western blot of the wild-type hZIP4 (WT) and the P298A and L300A double mutant. The glycosylated (upper band) and un-glycosylated (lower band) proteins are indicated by arrows. The surface expression level is indicated by the surface bound anti-HA antibody with three replicates. β-actin was detected using anti-β-actin antibody in western blot as loading control. bottom: zinc transport kinetics using the Hill model. The units of _K_m and _V_max are μM and pmol min−1 mg−1, respectively. Note that the kinetic parameters of the PL mutant were not measured because the activity is too low.
Figure 4. The role of the HRD in zinc transport.
(a) Zinc uptake assay and curve fitting of hZIP4, hZIP4-ΔHRD and hZIP4-ΔECD expressed in HEK293T cells. left: the raw experimental data; right: the processed data. Each data point represents the mean of three replicates. The error bars indicate 1±s.d. The results of curve fitting using the Hill model are shown in the Table 1 (c). (b) Western blot of hZIP4, hZIP4-ΔHRD and hZIP4-ΔECD expressed in HEK293T cells and surface expression levels are indicated by the surface bound anti-HA antibody with three replicates. β-actin was detected using anti-β-actin antibody in western blot as loading control. (c) Zinc transport kinetic parameters. The shown data are results of one out of three independent experiments. The data from the other two experiments are shown in Supplementary Fig. 8c,d. The units of _K_m and _V_max are μM and pmol min−1 mg−1, respectively. (d) Statistical analysis of the normalized _V_maxs (_V_max, n) from three independent experiments. The Student's _t_-test was used to assess significance statistically. ***P<0.001 and *P<0.05.
Figure 5. The roles of the bridging region in ZIP4 biogenesis and zinc transport.
(a) The zoomed-in structure of the bridging region. The key residues are shown in stick mode and other involved residues are shown in line mode. The dashed lines indicate potential hydrogen bonds. In hZIP4, P202 (P195 in pZIP4), W266 (W262 in pZIP4), D275 (D271 in pZIP4) and Q303 (Q299 in pZIP4) are mutated into alanine. (b) Functional characterization of P202A and W266A. (c) Functional characterization of D275A and Q303A. In both b and c, the raw experimental data of zinc transport assay (left), and western blots of the mutants in HEK293T cells, surface expression detection and western blots of β-actin (right) are from one representative experiment and the data sets from the other two independent experiments are shown in Supplementary Fig. 8e–h. Zinc transport kinetic parameters and statistical analysis are shown in the tables at the bottom. The units of _K_m and _V_max are μM and pmol min−1 mg−1, respectively. For W262A, D275A and Q303A, their zinc transport activities are too low to be accurately determined using the current approach. See more detailed data processing in Experimental Procedure and the figure legend of Fig. 1.
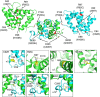
Figure 6. Mapping AE-causing mutations on the structure of pZIP4-ECD dimer.
The residues subjected to AE-causing mutations are highlighted in red. Mutations in human ZIP4 are shown in the brackets and the corresponding residues in pZIP4-ECD are indicated above the brackets. The zoomed-in structures in the proximity of these residues are shown in the small figures.
Figure 7. A structurally conserved fold of the ECDs in the LIV-1 subfamily.
(a) Comparison of the ECDs of the LIV-1 proteins. The phylogenetic tree of the LIV-1 proteins was generated by ClustalW2 (
http://www.ebi.ac.uk/Tools/msa/clustalw2/
) using the sequences of the full-length human proteins. The sequences of the ECDs from the nine LIV-1 proteins are depicted as rods. The signal peptide is coloured in grey, the predicted helix (by PSIPRED,
http://bioinf.cs.ucl.ac.uk/psipred/
) in red, the predicted strand in purple, the TMD in black, the histidine rich region in green and the signature PAL motif in blue. Black diamonds indicate universally conserved cysteine residue for each LIV-1 protein. (b) Structure-based sequence alignment of the ECDs of the human LIV-1 proteins. The generally conserved residues are shown in black blocks and the invariant residues are highlighted in red (the sequences of ZIP7 and ZIP13 are not included). The secondary structures from pZIP4-ECD are shown above the sequences. The predicted secondary structures of other LIV-1 proteins are shown below the sequence alignment. The National Center for Biotechnology Information (NCBI) reference sequence codes are: NP_570901 for ZIP4, NP_001128667 for ZIP5, NP_036451 for ZIP6, NP_001070984 for ZIP7, NP_001128618 for ZIP8, NP_065075 for ZIP10, NP_001138667 for ZIP12, NP_001121697 for ZIP13 and NP_001128625 for ZIP14. (c) The phylogenetic tree of the LIV-1 proteins (top) and the structural models of the ECDs of the LIV-1 proteins (bottom). The predicted unstructured sequences are shown by dotted lines. The length of the dotted line is not proportional to the number of the residues in this region and ZIP4, ZIP6 and ZIP10 contain one or multiple histidine-rich fragments within these regions. (d) Characterization of the purified pZIP14-ECD. top: sequence alignment of pZIP4 and pZIP14 in the proximity of the PAL motif. middle: size exclusion chromatography of the wild-type pZIP14-ECD and SDS–PAGE of pZIP14-ECD under reducing (Re) and non-reducing (Non-Re) conditions. bottom: size exclusion chromatography of the C147S mutant and SDS–PAGE of pZIP14-ECD under reducing and non-reducing conditions.
Similar articles
- The histidine-rich loop in the extracellular domain of ZIP4 binds zinc and plays a role in zinc transport.
Zhang T, Kuliyev E, Sui D, Hu J. Zhang T, et al. Biochem J. 2019 Jun 28;476(12):1791-1803. doi: 10.1042/BCJ20190108. Biochem J. 2019. PMID: 31164399 Free PMC article. - Structural and Functional Insights into Bacillus subtilis Sigma Factor Inhibitor, CsfB.
Martínez-Lumbreras S, Alfano C, Evans NJ, Collins KM, Flanagan KA, Atkinson RA, Krysztofinska EM, Vydyanath A, Jackter J, Fixon-Owoo S, Camp AH, Isaacson RL. Martínez-Lumbreras S, et al. Structure. 2018 Apr 3;26(4):640-648.e5. doi: 10.1016/j.str.2018.02.007. Epub 2018 Mar 8. Structure. 2018. PMID: 29526435 Free PMC article. - Characterization of the molecular properties of KtrC, a second RCK domain that regulates a Ktr channel in Bacillus subtilis.
Rocha R, Teixeira-Duarte CM, Jorge JMP, Morais-Cabral JH. Rocha R, et al. J Struct Biol. 2019 Mar 1;205(3):34-43. doi: 10.1016/j.jsb.2019.02.002. Epub 2019 Feb 10. J Struct Biol. 2019. PMID: 30753894 - The LIV-1 Subfamily of Zinc Transporters: From Origins to Present Day Discoveries.
Taylor KM. Taylor KM. Int J Mol Sci. 2023 Jan 9;24(2):1255. doi: 10.3390/ijms24021255. Int J Mol Sci. 2023. PMID: 36674777 Free PMC article. Review. - ZIP4: a promising early diagnostic and therapeutic targets for pancreatic cancer.
Tang Y, Guo S, Yu N, Li H. Tang Y, et al. Am J Cancer Res. 2024 Sep 25;14(9):4652-4664. doi: 10.62347/AVYM3477. eCollection 2024. Am J Cancer Res. 2024. PMID: 39417191 Free PMC article. Review.
Cited by
- Heterologous Expression of Full-Length and Truncated Human ZIP4 Zinc Transporter in Saccharomyces cerevisiae.
Liu Y, Bafaro EM, Dempski RE. Liu Y, et al. Biomolecules. 2022 May 21;12(5):726. doi: 10.3390/biom12050726. Biomolecules. 2022. PMID: 35625653 Free PMC article. - The zinc transporter ZIPT-7.1 regulates sperm activation in nematodes.
Zhao Y, Tan CH, Krauchunas A, Scharf A, Dietrich N, Warnhoff K, Yuan Z, Druzhinina M, Gu SG, Miao L, Singson A, Ellis RE, Kornfeld K. Zhao Y, et al. PLoS Biol. 2018 Jun 7;16(6):e2005069. doi: 10.1371/journal.pbio.2005069. eCollection 2018 Jun. PLoS Biol. 2018. PMID: 29879108 Free PMC article. - Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders.
Choi EK, Nguyen TT, Gupta N, Iwase S, Seo YA. Choi EK, et al. Sci Rep. 2018 Feb 16;8(1):3163. doi: 10.1038/s41598-018-21464-0. Sci Rep. 2018. PMID: 29453449 Free PMC article. - Zap70 Regulates TCR-Mediated Zip6 Activation at the Immunological Synapse.
Kim B, Kim HY, Lee WW. Kim B, et al. Front Immunol. 2021 Jul 29;12:687367. doi: 10.3389/fimmu.2021.687367. eCollection 2021. Front Immunol. 2021. PMID: 34394081 Free PMC article. - Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway.
Zhang T, Liu J, Fellner M, Zhang C, Sui D, Hu J. Zhang T, et al. Sci Adv. 2017 Aug 25;3(8):e1700344. doi: 10.1126/sciadv.1700344. eCollection 2017 Aug. Sci Adv. 2017. PMID: 28875161 Free PMC article.
References
- Nies D. H. & Silver S. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14, 186–199 (1995). - PubMed
- Lichten L. A. & Cousins R. J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 29, 153–176 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources