Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells - PubMed (original) (raw)
Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells
Jucimara Colombo et al. Oncol Lett. 2016 Jul.
Abstract
Liver cancer is the sixth most commonly occurring cancer globally, and the main histological type is hepatocellular carcinoma. This type of neoplasia has a poor prognosis due to a high rate of recurrence and intrahepatic metastasis, which are closely are closely associated with the angiogenic process. Vascular endothelial growth factor (VEGF), which is under the control of hypoxia inducible factor-1α (HIF-1α), stimulates the proliferation of endothelial cells and increases cell permeability, promoting the growth, spread and metastasis of tumors. Melatonin, the main hormone secreted by the pineal gland, may have a significant role in tumor suppression and has demonstrated antiangiogenic and antimetastatic effects. The aim of the present study was to analyze the cell viability, migration and invasion, as well as the expression of proangiogenic proteins VEGF and HIF-1α, in HepG2 hepatocarcinoma cells, following treatment with melatonin. Cells were cultured and cell viability was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of proangiogenic proteins VEGF and HIF-1α, under conditions of normoxia and hypoxia, was verified using immunocytochemistry and quantified by densitometry. The analysis of the processes of cell migration and invasion was performed in a Boyden chamber. The MTT assay revealed a reduction in cell viability (P=0.018) following treatment with 1 mM melatonin for 24 h. The expression of proangiogenic proteins VEGF and HIF-1α was reduced in cells treated with 1 mM melatonin for 24 h in normoxic (P<0.001) and hypoxic (P<0.001) conditions, compared with the control group and with induced hypoxia alone. The rate of cell migration and invasion was additionally reduced in cells treated with 1 mM melatonin for 48 h when compared with the control group (P=0.496). The results of the present study suggest that melatonin may have an antiproliferative, antiangiogenic and antimetastatic role in hepatocarcinoma cells and may present a novel therapeutic option for the treatment of liver cancer.
Keywords: HepG2; hypoxia-inducible factor 1α; invasion assay; liver cancer; melatonin; vascular endothelial growth factor.
Figures
Figure 1.
Analysis of cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay in liver cancer cells following 24 h of treatment with melatonin (1 nM, 100 nM, 10 µM and 1 mM). Data are expressed as a percentage of the control group, and presented as the mean ± standard deviation. *P<0.05 compared with the control.
Figure 2.
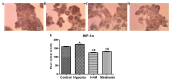
Immunocytochemistry of the protein HIF-1α, showing HepG2 cells (magnification, ×40) following 24 h of treatment with CoCl2 (100 µM) for induction of hypoxia and/or melatonin (1 mM). (A) Untreated cells (control group). (B) Cells with induced hypoxia. (C) Cells with induced hypoxia treated with melatonin. (D) Cells treated with melatonin alone. (E) Statistical analysis of protein expression of HIF-1α in the experimental groups. Data are presented as the mean optical density ± standard error, and are shown in arbitrary units. *P<0.05 compared with the control group. #P<0.05 compared with cells treated with CoCl2 alone. H + M, hypoxia and melatonin, M, melatonin; HIF-1α, hypoxia-inducible factor 1 α.
Figure 3.
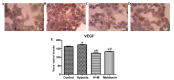
Immunocytochemistry of the protein VEGF, showing HepG2 cells (magnification, ×40) following 24 h of treatment with CoCl2 (100 µM) for induction of hypoxia and/or melatonin (1 mM). (A) Untreated cells (control group). (B) Cells with induced hypoxia. (C) Cells with induced hypoxia treated with melatonin. (D) Cells treated with melatonin alone. (E) Statistical analysis of protein expression of VEGF in the experimental groups. Data are presented as the mean optical density ± standard error, and are shown in arbitrary units. *P<0.05 compared with the control group. #P<0.05 compared with cells treated with CoCl2 alone. H+M, hypoxia and melatonin; M, melatonin; VEGF, vascular endothelial growth factor.
Figure 4.
Migration and invasion rate of the HepG2 cell line following 48 h of treatment with 1 mM melatonin. (A) Comparison between positive and negative control. (B) Comparison between the control with 10% fetal bovine serum without treatment and cells treated with 1 mM melatonin. Data are presented as the mean ± standard deviation. *P<0.05.
Similar articles
- Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin.
Goncalves Ndo N, Rodrigues RV, Jardim-Perassi BV, Moschetta MG, Lopes JR, Colombo J, Zuccari DA. Goncalves Ndo N, et al. Anticancer Agents Med Chem. 2014;14(9):1302-11. doi: 10.2174/1871520614666140812110246. Anticancer Agents Med Chem. 2014. PMID: 25323035 - Targeting HIF-1α and VEGF by lentivirus-mediated RNA interference reduces liver tumor cells migration and invasion under hypoxic conditions.
Chen J, Lai L, Liu S, Zhou C, Wu C, Huang M, Lin Q. Chen J, et al. Neoplasma. 2016;63(6):934-940. doi: 10.4149/neo_2016_612. Neoplasma. 2016. PMID: 27565331 - Melatonin Regulates Angiogenic Factors under Hypoxia in Breast Cancer Cell Lines.
Jardim-Perassi BV, Lourenço MR, Doho GM, Grígolo IH, Gelaleti GB, Ferreira LC, Borin TF, Moschetta MG, Pires de Campos Zuccari DA. Jardim-Perassi BV, et al. Anticancer Agents Med Chem. 2016;16(3):347-58. doi: 10.2174/1871520615666150511094201. Anticancer Agents Med Chem. 2016. PMID: 25963143 - Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review.
Vriend J, Reiter RJ. Vriend J, et al. Biochim Biophys Acta. 2016 Apr;1865(2):176-83. doi: 10.1016/j.bbcan.2016.02.004. Epub 2016 Feb 17. Biochim Biophys Acta. 2016. PMID: 26899267 Review. - Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence.
Goradel NH, Asghari MH, Moloudizargari M, Negahdari B, Haghi-Aminjan H, Abdollahi M. Goradel NH, et al. Toxicol Appl Pharmacol. 2017 Nov 15;335:56-63. doi: 10.1016/j.taap.2017.09.022. Epub 2017 Sep 30. Toxicol Appl Pharmacol. 2017. PMID: 28974455 Review.
Cited by
- Melatonin as an anti-inflammatory agent in radiotherapy.
Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S. Najafi M, et al. Inflammopharmacology. 2017 Aug;25(4):403-413. doi: 10.1007/s10787-017-0332-5. Epub 2017 Mar 2. Inflammopharmacology. 2017. PMID: 28255737 Review. - Melatonin as a potential inhibitory agent in head and neck cancer.
Yeh CM, Su SC, Lin CW, Yang WE, Chien MH, Reiter RJ, Yang SF. Yeh CM, et al. Oncotarget. 2017 Aug 9;8(52):90545-90556. doi: 10.18632/oncotarget.20079. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163852 Free PMC article. Review. - Melatonin and its mechanism of action in the female reproductive system and related malignancies.
Ezzati M, Velaei K, Kheirjou R. Ezzati M, et al. Mol Cell Biochem. 2021 Aug;476(8):3177-3190. doi: 10.1007/s11010-021-04151-z. Epub 2021 Apr 17. Mol Cell Biochem. 2021. PMID: 33864572 Review. - Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects.
Samec M, Liskova A, Koklesova L, Zhai K, Varghese E, Samuel SM, Šudomová M, Lucansky V, Kassayova M, Pec M, Biringer K, Brockmueller A, Kajo K, Hassan STS, Shakibaei M, Golubnitschaja O, Büsselberg D, Kubatka P. Samec M, et al. Cancers (Basel). 2021 Jun 16;13(12):3018. doi: 10.3390/cancers13123018. Cancers (Basel). 2021. PMID: 34208645 Free PMC article. Review. - Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation.
Bu LJ, Yu HQ, Fan LL, Li XQ, Wang F, Liu JT, Zhong F, Zhang CJ, Wei W, Wang H, Sun GP. Bu LJ, et al. World J Gastroenterol. 2017 Feb 14;23(6):986-998. doi: 10.3748/wjg.v23.i6.986. World J Gastroenterol. 2017. PMID: 28246472 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources