Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk - PubMed (original) (raw)
Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk
Patty C Kandalepas et al. PLoS One. 2016.
Abstract
Melatonin is released from the pineal gland into the circulatory system at night in the absence of light, acting as "hormone of darkness" to the brain and body. Melatonin also can regulate circadian phasing of the suprachiasmatic nucleus (SCN). During the day-to-night transition, melatonin exposure advances intrinsic SCN neural activity rhythms via the melatonin type-2 (MT2) receptor and downstream activation of protein kinase C (PKC). The effects of melatonin on SCN phasing have not been linked to daily changes in the expression of core genes that constitute the molecular framework of the circadian clock. Using real-time RT-PCR, we found that melatonin induces an increase in the expression of two clock genes, Period 1 (Per1) and Period 2 (Per2). This effect occurs at CT 10, when melatonin advances SCN phase, but not at CT 6, when it does not. Using anti-sense oligodeoxynucleotides (α ODNs) to Per 1 and Per 2, as well as to E-box enhancer sequences in the promoters of these genes, we show that their specific induction is necessary for the phase-altering effects of melatonin on SCN neural activity rhythms in the rat. These effects of melatonin on Per1 and Per2 were mediated by PKC. This is unlike day-active non-photic signals that reset the SCN clock by non-PCK signal transduction mechanisms and by decreasing Per1 expression. Rather, this finding extends roles for Per1 and Per2, which are critical to photic phase-resetting, to a nonphotic zeitgeber, melatonin, and suggest that the regulation of these clock gene transcripts is required for clock resetting by diverse regulatory cues.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
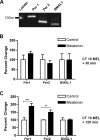
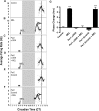
Fig 1. At CT 10, melatonin induces of Per1 and Per2 transcription by 120 min.
A) qPCR amplification products migrate at the predicted size and are distinguishable on an 8% polyacrylamide gel stained with ethidium bromide (Per1 = 113 bp, Per2 = 90 bp, BMAL1 = 79 bp). B) Melatonin has no significant effect on the expression levels of Per1, Per2, or Bmal1 mRNA 30 min following the initiation of treatment (p ≥ 0.05, Student’s T Test). C) Melatonin treatment significantly increases Per1 and Per2, but not Bmal1, transcripts, at 120 min. Data are shown as percent change of relative mRNA levels compared to control ± SEM, n = 3-4/condition. ***p ≤ 0.001 (Per1), *p ≤ 0.05 (Per2), p ≥ 0.05 (Bmal1), Student’s T-test.
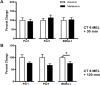
Fig 2. At CT 6, melatonin does not change the levels of Per1 and Per2 transcripts, although Bmal1 is reduced at 120 min.
Melatonin applied at CT 6 has no significant effect on the expression levels of Per 1, Per2, or Bmal1 mRNA after 30 min (A). After 120 min (B), only Bmal1 mRNA significantly decreases following initiation of melatonin treatment at CT 6. Data are shown as percent change of relative mRNA levels compared to control ± SEM, n = 3–9 /condition, p ≥ 0.05 (Per 1, Per 2), *p ≤ 0.05 (Bmal1), Student’s T-test.
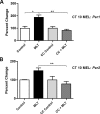
Fig 3. The PKC inhibitor, chelerythrine chloride, blocks the increase of Per1 and Per2 mRNA induced by melatonin applied at CT 10.
Pre-treatment with 0.25 mM of the PKC inhibitor, chelerythrine chloride, blocks the melatonin-induced increase in Per1 (A) and Per2 (B) transcripts after 120 min. Data are shown as percent change of relative mRNA levels compared to control ± SEM, n = 3/condition (** p ≤ 0.01, *p ≤ 0.05, 1-way ANOVA, Tukey’s post-hoc analysis). Controls were exposed to sham treatment lacking MEL. MEL = melatonin. CC = chelerythrine chloride.
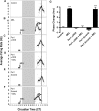
Fig 4. Per1 and Per2 αODN attenuate the expression of corresponding transcripts in the SCN.
2-h incubation of SCN slices with αODN results in a 45% decrease in Per1 transcripts (A) and a 60% decrease in Per2 transcripts (B) 4 h after initiation of treatment with the corresponding αODN. No change in GAPDH mRNA was evident following either treatment, which was used as a normalization control.
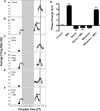
Fig 5. Per1 is required for melatonin to alter the phase of SCN neuronal activity rhythms at CT 10.
A) The spontaneous electrical activity rhythm in SCN brain slices peaks at CT 6.38 ± 0.13 in controls. The dotted line indicates the mean time-of-peak for untreated slices. Long, vertical boxes represent subjective night, CT 12–14. B) At CT 10, MEL (1 nM, 10 min) advances the electrical activity rhythm by 3.6 h ± 0.10 (n = 3). Arrow = time of melatonin treatment. C) Per1 αODN application from CT 8–10 has no significant effect on the time-of-peak electrical activity (n = 3). Small box = duration of ODN exposure. D) The MEL-induced phase advance is completely blocked by Per1 αODN (n = 3). E) Per1 missense ODN has no effect on the MEL-induced advance in time-of-peak electrical activity (n = 3). F) Per1 missense ODN does not block the MEL-induced phase advance at CT 10. G) Summary of the effects of Per1 ODN on MEL-induced phase advances at CT 10. **indicates statistically significant difference compared to controls (p ≤ 0.001) as determined by 1-way ANOVA with Tukey’s post-hoc analysis.
Fig 6. Per2 is required for melatonin to phase-shift SCN neuronal activity rhythms at CT 10.
A) The spontaneous electrical activity rhythm in SCN brain slices peaks at CT 6.38 ± 0.13 in control SCN (n = 3). B) At CT 10, MEL (1 nM, 10 min) advances the electrical activity rhythm by 3.6 h ± 0.10 (n = 3). C) Per2 αODN application from CT 8–10 has no effect on the mean time-of-peak electrical activity (n = 3). Small box = duration of ODN exposure. D) The MEL-induced phase advance is blocked completely by pre-incubation from CT 8–10 with Per2 αODN (n = 3). E) Pre-incubation with Per2 missense ODN has no effect on the MEL-induced advance in time-of-peak electrical activity (n = 3). F) Pre-incubation with Per2 missense ODN has no effect on the MEL-induced phase advance at CT 10 (n = 3). G) Summary of the effects of Per2 αODN pre-incubations on MEL-induced phase advances at CT 10. **indicates statistically significant differences (p ≤ 0.001) as determined by 1-way ANOVA with Tukey’s post-hoc analysis. Symbols as in Fig 5.
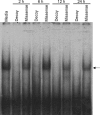
Fig 7. E-box decoy blocks binding at E-box sites in SCN 2.2 cells.
Electromobility shift assay of an E-box probe incubated with nuclear extracts of SCN 2.2 cells transfected with 1 μM E-box decoy or missense ODN. Media lane indicates non-transfected control. Arrow = retarded mobility of the E-box probe. This DNA-protein interaction is absent in SCN 2.2 cells transfected with the E-box decoy up to 24 h (n = 3).
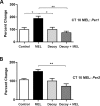
Fig 8. Melatonin-induced increases in Per1 and Per2 mRNAs are blocked by E-box decoy ODN.
Pre-treatment of SCN slices with E-box decoy ODN (1 μM), blocks the melatonin-induced increase in Per1 (A) and Per2 (B) transcripts after 120 min. qPCR data are shown as percent change of relative mRNA levels compared to control ± SEM, n = 3/condition (** p ≤ 0.01, *p ≤ 0.05, 1-way ANOVA, Tukey’s post-hoc analysis). MEL = melatonin.
Fig 9. E-box promoter motif is required for melatonin to shift SCN neuronal activity rhythms at CT 10.
A) The spontaneous electrical activity rhythm in SCN brain slices peaks at CT 6.38 ± 0.13 in controls (n = 3). The dotted line indicates the mean time-of-peak for untreated slices. Large, vertical boxes represent subjective night, CT 12–14. B) At CT 10, MEL (1 nM, 10 min) advances the electrical activity rhythm by 3.6 h ± 0.10 (n = 3). Arrow = time of melatonin treatment. C) E-box decoy ODN has no significant effect on the time-of-peak electrical activity (n = 3). Small box = duration of ODN exposure. D) The MEL-induced phase advance is blocked by the E-box decoy ODN (n = 3). E) Missense ODN has no effect on the MEL-induced advance in time-of-peak electrical activity (n = 3). F) Missense ODN does not block the MEL-induced phase advance at CT 10 (n = 3). G) Summary of the effects of ODN on MEL-induced phase advances at CT 10. **indicates statistically significant difference compared to controls (p ≤ 0.001) as determined by 1-way ANOVA with Tukey’s post- hoc analysis.
Similar articles
- Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei.
Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F. Poirel VJ, et al. Neuroscience. 2003;120(3):745-55. doi: 10.1016/s0306-4522(03)00344-0. Neuroscience. 2003. PMID: 12895514 - Dark pulse resetting of the suprachiasmatic clock in Syrian hamsters: behavioral phase-shifts and clock gene expression.
Mendoza JY, Dardente H, Escobar C, Pevet P, Challet E. Mendoza JY, et al. Neuroscience. 2004;127(2):529-37. doi: 10.1016/j.neuroscience.2004.05.026. Neuroscience. 2004. PMID: 15262341 - Photoperiod regulates multiple gene expression in the suprachiasmatic nuclei and pars tuberalis of the Siberian hamster (Phodopus sungorus).
Johnston JD, Ebling FJ, Hazlerigg DG. Johnston JD, et al. Eur J Neurosci. 2005 Jun;21(11):2967-74. doi: 10.1111/j.1460-9568.2005.04148.x. Eur J Neurosci. 2005. PMID: 15978008 - Melatonin feedback on clock genes: a theory involving the proteasome.
Vriend J, Reiter RJ. Vriend J, et al. J Pineal Res. 2015 Jan;58(1):1-11. doi: 10.1111/jpi.12189. Epub 2014 Nov 22. J Pineal Res. 2015. PMID: 25369242 Review. - Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals.
Challet E. Challet E. Endocrinology. 2007 Dec;148(12):5648-55. doi: 10.1210/en.2007-0804. Epub 2007 Sep 27. Endocrinology. 2007. PMID: 17901231 Review.
Cited by
- Melatonin receptors: molecular pharmacology and signalling in the context of system bias.
Cecon E, Oishi A, Jockers R. Cecon E, et al. Br J Pharmacol. 2018 Aug;175(16):3263-3280. doi: 10.1111/bph.13950. Epub 2017 Aug 17. Br J Pharmacol. 2018. PMID: 28707298 Free PMC article. Review. - Embryo transfers performed during daylight savings time led to reduced live birth rates in older patients.
Pelayo RA, Xu S, Walter JR. Pelayo RA, et al. J Assist Reprod Genet. 2023 Nov;40(11):2639-2647. doi: 10.1007/s10815-023-02920-x. Epub 2023 Sep 5. J Assist Reprod Genet. 2023. PMID: 37667016 Free PMC article. - Melatonin, mitochondria, and the cancer cell.
Proietti S, Cucina A, Minini M, Bizzarri M. Proietti S, et al. Cell Mol Life Sci. 2017 Nov;74(21):4015-4025. doi: 10.1007/s00018-017-2612-z. Epub 2017 Aug 7. Cell Mol Life Sci. 2017. PMID: 28785807 Free PMC article. Review. - Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression.
Valdés-Tovar M, Estrada-Reyes R, Solís-Chagoyán H, Argueta J, Dorantes-Barrón AM, Quero-Chávez D, Cruz-Garduño R, Cercós MG, Trueta C, Oikawa-Sala J, Dubocovich ML, Benítez-King G. Valdés-Tovar M, et al. Br J Pharmacol. 2018 Aug;175(16):3200-3208. doi: 10.1111/bph.14197. Epub 2018 Apr 17. Br J Pharmacol. 2018. PMID: 29512136 Free PMC article. Review. - In Silico, In Vitro, and In Vivo Analysis Identifies Endometrial Circadian Clock Genes in Recurrent Implantation Failure.
Zhai J, Li S, Hu J, Gao M, Sun Y, Chen ZJ, Giudice LC, Du Y. Zhai J, et al. J Clin Endocrinol Metab. 2021 Jun 16;106(7):2077-2091. doi: 10.1210/clinem/dgab119. J Clin Endocrinol Metab. 2021. PMID: 33619544 Free PMC article.
References
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI (2003) Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 8: d1093–1108. - PubMed
- Vanecek J, Pavlik A, Illnerova H (1987) Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res 435: 359–362. - PubMed
- Gillette MU, McArthur AJ (1996) Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res 73: 135–139. - PubMed
- McArthur AJ, Hunt AE, Gillette MU (1997) Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138: 627–634. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources