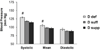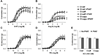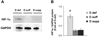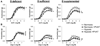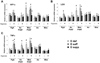Vitamin D controls resistance artery function through regulation of perivascular adipose tissue hypoxia and inflammation - PubMed (original) (raw)
Vitamin D controls resistance artery function through regulation of perivascular adipose tissue hypoxia and inflammation
Christopher J Pelham et al. J Mol Cell Cardiol. 2016 Sep.
Abstract
Vitamin D deficiency in human subjects is associated with hypertension, metabolic syndrome and related risk factors of cardiovascular diseases. Serum 25-hydroxyvitamin D levels correlate inversely with adiposity in obese and lean individuals. Bioactive vitamin D, or calcitriol, exerts anti-inflammatory effects on adipocytes, preadipocytes and macrophages in vitro. We tested the hypothesis that vitamin D deficiency alters the phenotype of perivascular adipose tissue (PVAT) leading to impaired function in resistance artery. To examine the effects of vitamin D and PVAT on vascular reactivity, myograph experiments were performed on arteries, with or without intact PVAT, from mice maintained on vitamin D-deficient, vitamin D-sufficient or vitamin D-supplemented diet. Systolic blood pressure was significantly increased in mice on vitamin D-deficient diet. Importantly, vitamin D deficiency enhanced angiotensin II-induced vasoconstriction and impaired the normal ability of PVAT to suppress contractile responses of the underlying mesenteric resistance artery to angiotensin II and serotonin. Furthermore, vitamin D deficiency caused upregulation of the mRNA expression of tumor necrosis factor-α, hypoxia-inducible factor-1α and its downstream target lysyl oxidase in mesenteric PVAT. Incubation of mesenteric arteries under hypoxic conditions impaired the anti-contractile effects of intact PVAT on those arteries from mice on vitamin D-sufficient diet. Vitamin D supplementation protected arteries against hypoxia-induced impairment of PVAT function. The protective effects of vitamin D against vascular dysfunction, hypertension and cardiovascular diseases may be mediated, at least in part, through regulation of inflammatory and hypoxia signaling pathways in PVAT.
Keywords: Hypoxia; Inflammation; Perivascular adipose tissue; Resistance artery; Vitamin D.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Financial and competing interests’ disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures
Figure 1. Vitamin D-deficient mice display increased blood pressure
Blood pressure was measured using the CODA non-invasive blood pressure system on mice maintained on the different vitamin D diets. Data represent means ± SE (n=8–10 mice). #p<0.05 D def or D supp vs. D suff.
Figure 2. Vitamin D deficiency eliminates anti-contractile effects of PVAT in mesenteric resistance arteries
Isometric tension was measured on second order mesenteric arteries from mice maintained on vitamin D-deficient diet (D def; squares), vitamin D-sufficient diet (D suff; circles) or vitamin D-supplemented diet (D supp; triangles). The mesenteric artery rings were either dissected free of PVAT (open symbols) or had the PVAT remaining intact (filled symbols). Dose-dependent contraction was assessed in response to 5-HT (A), angiotensin II (Ang II) (B), endothelin-1 (ET-1) (C), PE (D) or KCl (E). Data represent means ± SE (n=5 mice). *p<0.05 PVAT vs. no PVAT. #p<0.05 D def or D supp vs. D suff.
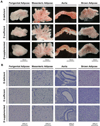
Figure 3. Effects of dietary vitamin D on morphology of different adipose depots
Representative images are shown for perigenital adipose tissue, mesenteric adipose tissue, aorta with intact PVAT and interscapular brown adipose tissue from mice maintained on the different vitamin D diets. (A) Images of the gross anatomy of tissue samples placed in a dish in Krebs buffer taken using a dissecting microscope (n=5–6 mice). (B) Images of sections from paraffin-embedded tissues stained with hematoxylin and eosin taken using a Nikon Eclipse Ci microscope (n=6–8 mice).
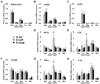
Figure 4. Vitamin D deficiency upregulates mediators of hypoxia and inflammation in mesenteric PVAT
Gene expression analysis was performed on samples of perigenital adipose tissue (PgA), aortic PVAT (Ao PVAT), mesenteric PVAT (Mes PVAT), aortic tissue (Ao) and mesenteric artery tissue (Mes) by quantitative real-time PCR. Relative expression of mRNA transcripts was determined after normalization to GAPDH. Data for adiponectin (A), leptin (B), HIF-1α (D), LOX (E), PTGIS (F), TNFα (G) and IL1β (H) are fold-change of vitamin D-deficient perigenital adipose tissue. UCP1 data (C) are fold change of vitamin D-deficient aortic PVAT. Data represent means ± SE (n=6 mice). #p<0.05 D def or D supp vs. D suff.
Figure 5. Vitamin D deficiency induces HIF-1α protein expression in mesenteric PVAT
Western blot analysis was performed on samples of mesenteric PVAT. A representative image of a blot is shown (A). Relative expression of HIF-1α protein was determined after normalization to GAPDH (B). Data are fold-change of vitamin D-deficient mesenteric PVAT. Data represent means ± SE (n=5 mice). #p<0.05 D def or D supp vs. D suff.
Figure 6. Hypoxic incubation inhibits anti-contractile effects of PVAT in normal mesenteric arteries
Isometric tension was measured on first order mesenteric arteries from mice maintained on the three vitamin D diets. The mesenteric artery rings were either dissected free of PVAT (open symbols) or had the PVAT remaining intact (filled symbols) and incubated for 24 hours under normoxic (squares) or hypoxic conditions (circles). Dose-dependent contraction was assessed in response to 5-HT (A) and Ang II (B) (see also Figure S2). Data represent means ± SE (n=4–5 mice). *p<0.05 PVAT vs. no PVAT. #p<0.05 D def or D supp vs. D suff.
Figure 7. Vitamin D supplementation negatively regulates expression of mediators of hypoxia and inflammation in adipose tissues
Gene expression analysis was performed on samples of perigenital adipose tissue (PgA), aortic PVAT (Ao PVAT), mesenteric PVAT (Mes PVAT), aortic tissue (Ao) and mesenteric artery tissue (Mes) by quantitative real-time PCR. Tissues were incubated for 24 hours under normoxic or hypoxic conditions Relative expression of mRNA transcripts was determined after normalization to GAPDH. Data for HIF-1α (A), LOX (B), and TNFα (C) are fold-change of vitamin D-deficient perigenital adipose tissue. Data represent means ± SE (n=3–4 mice). *p<0.05 hypoxic vs. normoxic. #p<0.05 D def or D supp vs. D suff.
Similar articles
- Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II.
Lu C, Su LY, Lee RM, Gao YJ. Lu C, et al. Eur J Pharmacol. 2010 May 25;634(1-3):107-12. doi: 10.1016/j.ejphar.2010.02.006. Epub 2010 Feb 13. Eur J Pharmacol. 2010. PMID: 20156432 - Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression.
Bhattacharya I, Drägert K, Albert V, Contassot E, Damjanovic M, Hagiwara A, Zimmerli L, Humar R, Hall MN, Battegay EJ, Haas E. Bhattacharya I, et al. Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2105-11. doi: 10.1161/ATVBAHA.112.301001. Epub 2013 Jul 18. Arterioscler Thromb Vasc Biol. 2013. PMID: 23868942 - Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: possible role for the leptin-inflammation-fibrosis-hypoxia axis.
Drosos I, Chalikias G, Pavlaki M, Kareli D, Epitropou G, Bougioukas G, Mikroulis D, Konstantinou F, Giatromanolaki A, Ritis K, Münzel T, Tziakas D, Konstantinides S, Schäfer K. Drosos I, et al. Clin Res Cardiol. 2016 Nov;105(11):887-900. doi: 10.1007/s00392-016-0996-7. Epub 2016 Jun 23. Clin Res Cardiol. 2016. PMID: 27337945 - Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease.
Lastra G, Manrique C. Lastra G, et al. Horm Mol Biol Clin Investig. 2015 Apr;22(1):19-26. doi: 10.1515/hmbci-2015-0010. Horm Mol Biol Clin Investig. 2015. PMID: 25941914 Review. - [Functional Relationship between Arterial Tissue and Perivascular Adipose Tissue in Metabolic Syndrome].
Kagota S, Iwata S, Maruyama K, Wakuda H, Shinozuka K. Kagota S, et al. Yakugaku Zasshi. 2016;136(5):693-7. doi: 10.1248/yakushi.15-00262-2. Yakugaku Zasshi. 2016. PMID: 27150921 Review. Japanese.
Cited by
- Role of Vitamin D in Cardiovascular Diseases.
Rai V, Agrawal DK. Rai V, et al. Endocrinol Metab Clin North Am. 2017 Dec;46(4):1039-1059. doi: 10.1016/j.ecl.2017.07.009. Epub 2017 Sep 29. Endocrinol Metab Clin North Am. 2017. PMID: 29080634 Free PMC article. Review. - Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway.
Zhu X, Zhang HW, Chen HN, Deng XJ, Tu YX, Jackson AO, Qing JN, Wang AP, Patel V, Yin K. Zhu X, et al. Acta Pharmacol Sin. 2019 Jan;40(1):46-54. doi: 10.1038/s41401-018-0068-9. Epub 2018 Jul 12. Acta Pharmacol Sin. 2019. PMID: 30002491 Free PMC article. - The Potential Effect of Intravenous Calcitriol on the Ischemia-Reperfusion Process and Inflammatory Biomarkers in Patients Following Percutaneous Coronary Intervention (PCI).
Dastan F, Salamzadeh J, Alipour-Parsa S, Sharif Kashani B, Hashempour MM. Dastan F, et al. Iran J Pharm Res. 2019 Fall;18(Suppl1):282-290. doi: 10.22037/ijpr.2019.112469.13778. Iran J Pharm Res. 2019. PMID: 32802107 Free PMC article. - Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model.
Süli A, Magyar P, Vezér M, Bányai B, Szekeres M, Sipos M, Mátrai M, Hetthéssy JR, Dörnyei G, Ács N, Horváth EM, Nádasy GL, Várbíró S, Török M. Süli A, et al. Int J Mol Sci. 2023 Nov 21;24(23):16577. doi: 10.3390/ijms242316577. Int J Mol Sci. 2023. PMID: 38068901 Free PMC article. - Vitamin D deficiency causes inward hypertrophic remodeling and alters vascular reactivity of rat cerebral arterioles.
Pál É, Hadjadj L, Fontányi Z, Monori-Kiss A, Mezei Z, Lippai N, Magyar A, Heinzlmann A, Karvaly G, Monos E, Nádasy G, Benyó Z, Várbíró S. Pál É, et al. PLoS One. 2018 Feb 6;13(2):e0192480. doi: 10.1371/journal.pone.0192480. eCollection 2018. PLoS One. 2018. PMID: 29408903 Free PMC article.
References
- Trayhurn P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr. 2014;34:207–236. - PubMed
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009 Mar 31;119(12):1661–1670. - PubMed
- Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013 Jul 9;62(2):128–135. - PMC - PubMed
- Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012 Jul;20(7):1444–1448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical