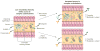Diet-microbiota interactions as moderators of human metabolism - PubMed (original) (raw)
Review
Diet-microbiota interactions as moderators of human metabolism
Justin L Sonnenburg et al. Nature. 2016.
Abstract
It is widely accepted that obesity and associated metabolic diseases, including type 2 diabetes, are intimately linked to diet. However, the gut microbiota has also become a focus for research at the intersection of diet and metabolic health. Mechanisms that link the gut microbiota with obesity are coming to light through a powerful combination of translation-focused animal models and studies in humans. A body of knowledge is accumulating that points to the gut microbiota as a mediator of dietary impact on the host metabolic status. Efforts are focusing on the establishment of causal relationships in people and the prospect of therapeutic interventions such as personalized nutrition.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1. Interactions between the diet and the gut microbiota dictate the production of short-chain fatty acids
Dietary fibre is a source of complex carbohydrates, which are required for the production of short-chain fatty acids such as acetate, butyrate and propionate. When the diversity of the microbiota is high and the diet contains many types of complex carbohydrates (top right), a relatively high percentage of complex carbohydrates will be accessible to the microbiota. But when the diversity of the microbiota is low and the diet contains many types of complex carbohydrates (left), only a low percentage of these complex carbohydrates are accessible to the microbiota. If the fibre composition of the diet is matched to the needs of a low-diversity microbiota (bottom right) by limiting the types of complex carbohydrate that are available, the levels of production of certain short-chain fatty acids, such as propionate, might increase. However, the diversity of the microbiota will probably remain low and it might not be able to provide as many functions as a diverse microbiota. Consumption of a complex diet (top right) might result in increased levels of production of multiple types of short-chain fatty acids and helps to recruit additional diversity to the gut microbiota. The level of propionate production is correlated with the abundance of Bacteroides species in the gut, which is consistent with the involvement of these bacteria in the production of propionate. Fermentation of fibre in the colon has been shown to decrease pH levels, which can help to increase the diversity of the gut microbiota or results in the reinforcement by certain taxa of a pH that favours their own growth–.
Figure 2. Mechanisms of signalling from the gut microbiota to the host
The gut microbiota interacts with dietary components and metabolites to form bioactive metabolites that signal to the host through distinct mechanisms. Short-chain fatty acids that are produced by the fermentation of fibre are an important source of energy (ATP) for colonocytes. They are also a substrate for gluconeogenesis, which modulates central metabolism, and are involved in signalling to the host by inhibiting histone deacetylase (HDAC) or by activating G-protein-coupled receptors such as GPR41 and GPR43, which triggers the release of the hormone glucagon-like peptide-1. The primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) are metabolized into the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), which activates signalling to the host through the G-protein-coupled bile acid receptor 1 (GPBAR1; also known as TGR5). Tauro-β-muricholic acid (TβMCA) is deconjugated into β-muricholic acid (βMCA; not shown), which alleviates the inhibition of the farnesoid X-activated receptor (FXR; also known as the bile acid receptor) by TβMCA. Microbially produced endotoxins (also known as lipopolysaccharides) are taken up into chylomicrons that are formed from dietary saturated fats and subsequently they promote inflammation in the host that induces insulin resistance. L-Carnitine and choline, compounds that are found in red meat, are metabolized into TMAs that are oxidized further into TMAO by the enzyme flavin-containing monooxygenase 3 (FMO3) in the liver (inset).
Figure 3. Strategies for modulating the gut microbiota to improve human health
a, The collection and comparison of multi-omics data from healthy people and those who are affected by metabolic disorders will implicate various genes, pathways and molecules as potential targets for intervention. Relevant experimental models (in vitro, organoid or animal models) are then used to elucidate underlying mechanisms and to pilot therapeutic approaches to modulating the gut microbiota, which lay the foundations for intervention studies or drug trials in humans. b, Studies in humans can also be a starting point for the identification of strategies to modulate the gut microbiota through components of the diet, which are generally considered to be ‘safe’ interventions. Data-processing algorithms, such as machine learning, can be used to identify aspects of the clinical profile of individuals (including data on the microbiota) that help to predict the response of others to dietary interventions. After validation of these predictive elements in independent cohorts, the best intervention can be determined and then implemented to improve human health. Such predictive elements can also be used to guide mechanistic studies in experimental models.
Similar articles
- [Gut microbiota and immune crosstalk in metabolic disease].
Burcelin R. Burcelin R. Biol Aujourdhui. 2017;211(1):1-18. doi: 10.1051/jbio/2017008. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682223 Review. French. - Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies.
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Aron-Wisnewsky J, et al. Gastroenterology. 2021 Jan;160(2):573-599. doi: 10.1053/j.gastro.2020.10.057. Epub 2020 Nov 27. Gastroenterology. 2021. PMID: 33253685 Review. - Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes.
Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H. Scheithauer TPM, et al. Front Immunol. 2020 Oct 16;11:571731. doi: 10.3389/fimmu.2020.571731. eCollection 2020. Front Immunol. 2020. PMID: 33178196 Free PMC article. Review. - Interplay of Gut Microbiota, Probiotics in Obesity: A Review.
Pothuraju R, Sharma RK. Pothuraju R, et al. Endocr Metab Immune Disord Drug Targets. 2018;18(3):212-220. doi: 10.2174/1871530318666180131092203. Endocr Metab Immune Disord Drug Targets. 2018. PMID: 29384067 Review. - Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids.
Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, Li X, Ichimura A, Irie J, Sugimoto Y, Mizutani T, Sugawara T, Miki T, Ogawa J, Drucker DJ, Arita M, Itoh H, Kimura I. Miyamoto J, et al. Nat Commun. 2019 Sep 5;10(1):4007. doi: 10.1038/s41467-019-11978-0. Nat Commun. 2019. PMID: 31488836 Free PMC article.
Cited by
- Changes in social environment impact primate gut microbiota composition.
Pearce CS, Bukovsky D, Douchant K, Katoch A, Greenlaw J, Gale DJ, Nashed JY, Brien D, Kuhlmeier VA, Sabbagh MA, Blohm G, De Felice FG, Pare M, Cook DJ, Scott SH, Munoz DP, Sjaarda CP, Tusche A, Sheth PM, Winterborn A, Boehnke S, Gallivan JP. Pearce CS, et al. Anim Microbiome. 2024 Nov 13;6(1):66. doi: 10.1186/s42523-024-00355-y. Anim Microbiome. 2024. PMID: 39538341 Free PMC article. - Gut microbiota: a crucial player in the combat against tuberculosis.
Lin J, Chen D, Yan Y, Pi J, Xu J, Chen L, Zheng B. Lin J, et al. Front Immunol. 2024 Oct 22;15:1442095. doi: 10.3389/fimmu.2024.1442095. eCollection 2024. Front Immunol. 2024. PMID: 39502685 Free PMC article. Review. - Eubacterium siraeum suppresses fat deposition via decreasing the tyrosine-mediated PI3K/AKT signaling pathway in high-fat diet-induced obesity.
Lai X, Liu S, Miao J, Shen R, Wang Z, Zhang Z, Gong H, Li M, Pan Y, Wang Q. Lai X, et al. Microbiome. 2024 Oct 30;12(1):223. doi: 10.1186/s40168-024-01944-4. Microbiome. 2024. PMID: 39478562 Free PMC article. - Seasonal variations in composition and function of gut microbiota in grazing yaks: Implications for adaptation to dietary shift on the Qinghai-Tibet plateau.
Wang X, Guo T, Zhang Q, Zhao N, Hu L, Liu H, Xu S. Wang X, et al. Ecol Evol. 2024 Oct 22;14(10):e70337. doi: 10.1002/ece3.70337. eCollection 2024 Oct. Ecol Evol. 2024. PMID: 39440203 Free PMC article. - The role of dietary inflammatory index in metabolic diseases: the associations, mechanisms, and treatments.
Xu J, Xie L, Fan R, Shi X, Xu W, Dong K, Ma D, Yan Y, Zhang S, Sun N, Huang G, Gao M, Yu X, Wang M, Wang F, Chen J, Tao J, Yang Y. Xu J, et al. Eur J Clin Nutr. 2024 Oct 21. doi: 10.1038/s41430-024-01525-6. Online ahead of print. Eur J Clin Nutr. 2024. PMID: 39433856 Review.
References
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nature Clin Pract Endocrinol Metab. 2009;5:150–159. - PubMed
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical