Free Fatty Acids Differentially Downregulate Chemokines in Liver Sinusoidal Endothelial Cells: Insights into Non-Alcoholic Fatty Liver Disease - PubMed (original) (raw)
Free Fatty Acids Differentially Downregulate Chemokines in Liver Sinusoidal Endothelial Cells: Insights into Non-Alcoholic Fatty Liver Disease
Rachel H McMahan et al. PLoS One. 2016.
Erratum in
- Correction: Free Fatty Acids Differentially Downregulate Chemokines in Liver Sinusoidal Endothelial Cells: Insights into Non-Alcoholic Fatty Liver Disease.
McMahan RH, Porsche CE, Edwards MG, Rosen HR. McMahan RH, et al. PLoS One. 2016 Dec 8;11(12):e0168301. doi: 10.1371/journal.pone.0168301. eCollection 2016. PLoS One. 2016. PMID: 27930747 Free PMC article.
Abstract
Non-alcoholic fatty liver disease is a prevalent problem throughout the western world. Liver sinusoidal endothelial cells (LSEC) have been shown to play important roles in liver injury and repair, but their role in the underlying pathogenetic mechanisms of non-alcoholic fatty liver disease remains undefined. Here, we evaluated the effects of steatosis on LSEC gene expression in a murine model of non-alcoholic fatty liver disease and an immortalized LSEC line. Using microarray we identified distinct gene expression profiles following exposure to free fatty acids. Gene pathway analysis showed a number of differentially expressed genes including those involved in lipid metabolism and signaling and inflammation. Interestingly, in contrast to hepatocytes, fatty acids led to decreased expression of pro-inflammatory chemokines including CCL2 (MCP-1), CXCL10 and CXCL16 in both primary and LSEC cell lines. Chemokine downregulation translated into a significant inhibition of monocyte migration and LSECs isolated from steatotic livers demonstrated a similar shift towards an anti-inflammatory phenotype. Overall, these pathways may represent a compensatory mechanism to reverse the liver damage associated with non-alcoholic fatty liver disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
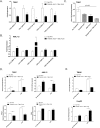
Fig 1. Gene expression analysis of TSEC treated with FFA.
TSECs were treated with 0.33mM OA and 0.33mM PA for 16 hours. RNA was isolated from cells and microarray analysis of triplicate wells was performed using IPA (Qiagen). (A) Heat map of the top 20 up-regulated and down-regulated genes in the FFA treated LSEC compared to untreated cells. Individual samples are given on the rows, while genes are listed by column. Gene expression is normalized to the mean and color-scaled by standard deviations above (red) and below (green) the average expression in all six independent samples. (B) Upregulation of genes involved in lipid metabolism genes (red) and the predicted activated pathways (orange) in TSEC treated with FFA. (C) Predicted activation of PPARα based on Ingenuity upstream regulator analysis. Upregulated genes downstream of PPARα are shown (red).
Fig 2. KEGG pathways in FFA treated TSEC.
Relevant enriched pathways and the involved genes were determined by analysis of all genes with a p value <0.05 and a fold-change > 1.25 within the KEGG pathway database.
Fig 3. Downregulation of chemokines by LSEC in response to treatment with FFA.
TSEC (A) and AML12 (B) were cultured with 0.33mM OA and/or 0.33mM PA for 16 hours. RNA was isolated from cells and levels of the indicated chemokine were determined by quantitative RT PCR. (C) TSEC were treated with 0.33mM of the indicated FFA for 16 hours and CCL2 expression was determined by quantitative RT PCR. (D) TSEC and AML12 were treated with 0.33mM OA and 0.33mM PA for 16 hours followed by 6 hour stimulation with 100ng/ml LPS. CCL2 gene expression (top) and protein production (bottom) were measured. (E) The human LSEC (TMNK) and hepatocyte (HepG2) cell lines were stimulated with FFA and LPS as in (D) and CCL2 gene expression was measured by quantitative PCR. Plots represent the mean +/- SE of three experiments. *p<0.05.
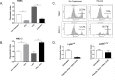
Fig 4. FFA inhibits the migration of monocytes in vitro.
Murine monocytes were isolated from the bone marrow of C56Bl6/J mice and co-cultured for 2 hours in transwell plates with TSEC (A) or AML12 (B) either resting or pre-treated with FFA and LPS for 18 hours. Cells were stained with the monocyte/macrophage markers CD11b, F/480 and Ly6C and the chemotactic index was calculated (#migrated cells in treatment wells/# migrated cell in the control well). (C) Representative histograms showing Ly6C expression on migrated cells. (D) The chemotactic index of Ly6C high and Ly6C low cells towards resting or FFA-treated TSEC. Plots represent the mean +/- SE of three experiments. *p<0.05.
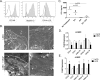
Fig 5. Phenotype of LSEC from normal and DIO mice.
A) Primary LSEC (CD45-CD146+) isolated from normal C57Bl6 lean mice were analyzed for CD146 and CD31 expression and uptake of DiI-labeled Ac-LDL by flow cytometry. (B) Primary LSEC isolated from C57Bl6 lean mice were cultured with the indicated FFA for 24hours. CCL2 levels in the supernatant were measured by ELISA. Graphs represent the mean +/- SE from 3 mice. Primary LSEC (CD45-CD146+) (C) and LSEC depleted non-parenchymal cells (CD45+CD146-) (D) were isolated from mice fed a low fat diet (NCD) and obese mice fed a high fat diet (HFD) for 12 weeks and gene expression for chemokines was evaluated by quantitative qPCR. Graphs represent the mean +/- SE from 5–7 mice. *p<0.05.
Similar articles
- Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines.
Schrage A, Wechsung K, Neumann K, Schumann M, Schulzke JD, Engelhardt B, Zeitz M, Hamann A, Klugewitz K. Schrage A, et al. Hepatology. 2008 Oct;48(4):1262-72. doi: 10.1002/hep.22443. Hepatology. 2008. PMID: 18697212 - LSEC Fenestrae Are Preserved Despite Pro-inflammatory Phenotype of Liver Sinusoidal Endothelial Cells in Mice on High Fat Diet.
Kus E, Kaczara P, Czyzynska-Cichon I, Szafranska K, Zapotoczny B, Kij A, Sowinska A, Kotlinowski J, Mateuszuk L, Czarnowska E, Szymonski M, Chlopicki S. Kus E, et al. Front Physiol. 2019 Feb 12;10:6. doi: 10.3389/fphys.2019.00006. eCollection 2019. Front Physiol. 2019. PMID: 30809151 Free PMC article. - Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease.
Hammoutene A, Rautou PE. Hammoutene A, et al. J Hepatol. 2019 Jun;70(6):1278-1291. doi: 10.1016/j.jhep.2019.02.012. Epub 2019 Feb 21. J Hepatol. 2019. PMID: 30797053 Review. - Changes induced by non-alcoholic fatty liver disease in liver sinusoidal endothelial cells and hepatocytes: spectroscopic imaging of single live cells at the subcellular level.
Kochan K, Kus E, Szafraniec E, Wislocka A, Chlopicki S, Baranska M. Kochan K, et al. Analyst. 2017 Oct 9;142(20):3948-3958. doi: 10.1039/c7an00865a. Analyst. 2017. PMID: 28944783 - Decoding cell death signals in liver inflammation.
Brenner C, Galluzzi L, Kepp O, Kroemer G. Brenner C, et al. J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
- Macrophage functional diversity in NAFLD - more than inflammation.
Barreby E, Chen P, Aouadi M. Barreby E, et al. Nat Rev Endocrinol. 2022 Aug;18(8):461-472. doi: 10.1038/s41574-022-00675-6. Epub 2022 May 9. Nat Rev Endocrinol. 2022. PMID: 35534573 Review. - Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role.
Gupta H, Min BH, Ganesan R, Gebru YA, Sharma SP, Park E, Won SM, Jeong JJ, Lee SB, Cha MG, Kwon GH, Jeong MK, Hyun JY, Eom JA, Park HJ, Yoon SJ, Choi MR, Kim DJ, Suk KT. Gupta H, et al. Biomedicines. 2022 Feb 25;10(3):550. doi: 10.3390/biomedicines10030550. Biomedicines. 2022. PMID: 35327352 Free PMC article. Review. - Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review.
Nasiri-Ansari N, Androutsakos T, Flessa CM, Kyrou I, Siasos G, Randeva HS, Kassi E, Papavassiliou AG. Nasiri-Ansari N, et al. Cells. 2022 Aug 12;11(16):2511. doi: 10.3390/cells11162511. Cells. 2022. PMID: 36010588 Free PMC article. Review. - Endothelial dysfunction in pathological processes of chronic liver disease during aging.
Wan Y, Li X, Slevin E, Harrison K, Li T, Zhang Y, Klaunig JE, Wu C, Shetty AK, Dong XC, Meng F. Wan Y, et al. FASEB J. 2022 Jan;36(1):e22125. doi: 10.1096/fj.202101426R. FASEB J. 2022. PMID: 34958687 Free PMC article. Review. - Crosstalk Between Liver Macrophages and Surrounding Cells in Nonalcoholic Steatohepatitis.
Li H, Zhou Y, Wang H, Zhang M, Qiu P, Zhang M, Zhang R, Zhao Q, Liu J. Li H, et al. Front Immunol. 2020 Jun 24;11:1169. doi: 10.3389/fimmu.2020.01169. eCollection 2020. Front Immunol. 2020. PMID: 32670278 Free PMC article. Review.
References
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023. 10.1002/hep.25762 - DOI - PubMed
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. (2007) A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous