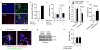Transfer of mitochondria from astrocytes to neurons after stroke - PubMed (original) (raw)
Transfer of mitochondria from astrocytes to neurons after stroke
Kazuhide Hayakawa et al. Nature. 2016.
Erratum in
- Corrigendum: Transfer of mitochondria from astrocytes to neurons after stroke.
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Hayakawa K, et al. Nature. 2016 Nov 3;539(7627):123. doi: 10.1038/nature19805. Epub 2016 Sep 14. Nature. 2016. PMID: 27629516 No abstract available.
Abstract
Neurons can release damaged mitochondria and transfer them to astrocytes for disposal and recycling. This ability to exchange mitochondria may represent a potential mode of cell-to-cell signalling in the central nervous system. Here we show that astrocytes in mice can also release functional mitochondria that enter neurons. Astrocytic release of extracellular mitochondrial particles was mediated by a calcium-dependent mechanism involving CD38 and cyclic ADP ribose signalling. Transient focal cerebral ischaemia in mice induced entry of astrocytic mitochondria into adjacent neurons, and this entry amplified cell survival signals. Suppression of CD38 signalling by short interfering RNA reduced extracellular mitochondria transfer and worsened neurological outcomes. These findings suggest a new mitochondrial mechanism of neuroglial crosstalk that may contribute to endogenous neuroprotective and neurorecovery mechanisms after stroke.
Conflict of interest statement
Competing Interests: The authors declare they have no competing financial interest.
Figures
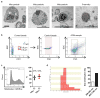
Extended Data Figure 1. Astrocytic mitochondria particle detection
a, Electron microscopic analysis demonstrated that mitochondria were detected within extracellular astrocyte-derived particles. Free mitochondria were also found in astrocyte-conditioned medium.b, In FACS analysis, control beads were used to gate population ranging in size from 500 nm to 900 nm. c, In astrocyte-derived conditioned media, approximately 53% of particles in the range of size were positive for functional mitochondria (n=5). d, After FACS analysis to isolate extracellular mitochondria fraction from astrocyte-conditioned media, particle size was measured with qNano analysis. Consistent with electron microscope analysis, a range of size distributions were observed (~25%: 300 – 400 nm, ~75%: 400 – 1100 nm). All values are mean +/− SEM.
Extended Data Figure 2. Characteristics of astrocytic mitochondria particle in FACS analysis
a, Mitochondrial particles were identified by FACS.b, Of these mitochondrial particles, FACS analysis identified that approximately 79% and 43% of particles express β1-integrin and CD63, respectively (n=4). cADPR (1 μM) did not appear to affect these distributions (n=4). All values are mean +/− SEM.
Extended Data Figure 3. Production of astrocytic mitochondria particle in a Ca2+-dependent mechanism
a, The known CD38 downstream signal, cADPR increased intracellular calcium shown in Fluo-4 intensity in a concentration-dependent manner (n=3). b, Intracellular ATP in astrocytes was upregulated by cADPR stimulation (n=4). **P<0.01 vs cADPR 0 μM. c, To measure ATP levels in extracellular particles, astrocyte-conditioned media were collected and large debris were excluded by centrifugation and filtration using 1.2 μm filter. Following another centrifugation at 20,000g for 30 min, each 100 μl from top or bottom fractions were used for ATP assay.d, The bottom fraction had higher ATP content, and cADPR (1 μM) increased ATP content in this bottom fraction (n= 6 or 8). e, cADPR-induced extracellular ATP levels within extracellular particles was diminished by intracellular calcium blocker, BAPTA-AM (n= 4 or 6). All values are mean +/− SEM.
Extended Data Figure 4. Summary of experiment on Figure 2c
a, We repeated the experiment in Fig. 2c with n=4 independent primary cultures per group. Similar results were obtained. The extracellular mitochondria-depleted astrocyte media (mdACM) group was significantly different compared to the ACM group. Furthermore, in this repeated experiment, there was also statistical significance between controls (OGD-damaged neurons alone) versus those treated with mitochondria-containing astrocyte media (ACM), and there was no statistically significant worsening when comparing control versus mitochondria-depleted groups (mdACM). Taken together, these two separate experiments suggest a modest but statistically significant neuroprotection induced by astrocyte-derived mitochondria. All values are mean +/− SEM. b, Mitotracker Red CMXRos (200 nM) was incubated without astrocytes to obtain no-cell-derived media (negative control). Media was collected and further incubated with neurons following oxygen-glucose deprivation. After 24 hours, there was no mitochondrial signal observed. Scale: 100 μm.
Extended Data Figure 5. Role of astrocytic CD38 in mitochondria transfer during starvation in vitro
a, Immunocytochemistry in neuron-astrocyte co-cultures demonstrated that CD38 was primarily expressed within astrocytes.b, Extracellular ATP levels were higher in media collected from neurons co-cultured with astrocytes compared to neuron-alone cultures alone (n=9 or 11). c, After serum/glucose starvation, neurons were significantly damaged, as expected. But neurons co-cultured with astrocytes were protected (n=6 or 4). d, CD38 suppression with siRNA significantly decreased extracellular ATP levels in neuron-astrocyte co-culture, but CD38 suppression did not affect extracellular ATP level in neuron-alone cultures (n=9 or 6).e, Blockade of astrocytic CD38 with siRNA significantly increased LDH release (indicative of cell damage) in the co-culture, suggesting that CD38 may be important to maintain neuroglial homeostasis (n=6). f, Rat primary neurons were co-cultured with rat astrocytes. Immunocytochemistry showed that CD38 suppression with siRNA reduced astrocytic mitochondria (red) transfer into neurons compared to control. g, h, Western blot analysis indicated that CD38 suppression with siRNA can be successfully performed in astrocyte culture without affecting cell viability (n=4 or 3). All values are mean +/− SEM.
Extended Data Figure 6. Metabolic inhibition in astrocyte causes neuronal cell death and retards neurite outgrowth in vitro
a, Astrocytic aconitase was inhibited by fluorocitrate (FC) which disrupted astrocyte metabolism that was accompanied by SA-β-gal signal. b, Intracellular ATP was decreased in these metabolically-disrupted astrocytes (n=6). *P<0.05, **P<0.01 vs FC 0 mM. c, PI staining showed that fluorocitrate (0.5 mM) did not induce cell death in astrocytes.d, Metabolically-disrupted astrocytes significantly decreased mitochondrial membrane potential. Red: aggregated JC1, Green: monomer JC1. Scale: 20 μm. e, Rat cortical neurons were co-cultured with JC1-labeled astrocytes. After 24 hours co-culture, control astrocytes transferred mitochondria which had a high-membrane potential (aggregated JC1), but metabolically-disrupted astrocytes released and transferred dysfunctional mitochondria into neurons (n=3).f, Metabolically-disrupted astrocytes could not support neural viability under starvation in the co-culture (n=4).g, Co-culture between astrocytes and neurons was conducted for 48 hours to test neurite outgrowth. Immunocytochemistry showed that metabolically-disrupted astrocytes retarded neurite outgrowth and increased neuronal cell death (n=3). h, LDH assay indicated that fluorocitrate (0.5 mM) did not affect cell viability in either rat cortical astrocytes (n=4) or rat cortical neurons (n=4). All values are mean +/− SEM.
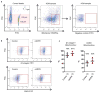
Extended Data Figure 7. FACS analysis using E17 FVB/N-Tg (GFAPGFP)14Mes/J transgenic mice
a, Cortical neurons were isolated from E17 FVB/N-Tg (GFAPGFP)14Mes/J transgenic mice. Immunocytochemistry showed that cultured neurons did not express either GFP or GFAP protein following oxygen-glucose deprivation, suggesting that stroke-like stress may not likely lead to “leakiness” in this astrocyte-specific GFP mouse.b, Brain cell suspension was prepared from FVB/N-Tg (GFAPGFP)14Mes/J mice subjected to transient ischemia, then FACS analysis was performed. c, Representative image before cell sorting.d, Purity after cell sorting. e, Either MAP2+/GFP- or MAP2+/GFP+ population was positive for DAPI as 92.5% or 85.9%, respectively. **f,**Western blot analysis demonstrated that both GFP-positive and negative neurons expressed mature neuron marker (neurofilament) but not neuronal stem cell marker (nestin). These data exclude the possibility that GFAP-positive cells included subsets of neuronal precursor cells that are known to also express GFAP.
Extended Data Figure 8. Effects of CD38 suppression with siRNA in vivo and in vitro
a, Western blot showed that CD38 expression was increased in peri-infarct cortex at days 1 to 7 after stroke.b, CD38 siRNA or a scrambled control was injected into lateral ventricles at 5 days after stroke. Western blot analysis confirmed that CD38 expression was successfully decreased in peri-infarct cortex at 7 days. c, In peri-infarct cortex, CD8 T cell and Iba1 positive microglia/macrophage were detected by immunohistochemistry. **d,**Quantification of the number of CD8 positive cells or Iba1 positive cells indicated that there was no difference between control siRNA and CD38 siRNA (n=6). All values are mean +/− SEM. **e,**Cultured rat cortical astrocytes were subjected to oxygen-glucose deprivation for 2 hours followed by treating with control siRNA or CD38 siRNA. Astrocyte cell morphology or GFAP expression was assessed by immunocytochemistry or western blot after 22-h reoxygenation.f, Morphology change was not clearly observed in cultured astrocytes suppressed CD38 with siRNA compared to control siRNA.g, Western blot analysis showed that CD38 was successfully decreased by siRNA transfection but GFAP expression was not clearly changed.
Extended Data Figure 9. Neuronal purity confirmed by FACS analysis in vivo
To be sure about our FACS findings, we used two different standard approaches that have been published in the literature (Bi et al, J Neurosci 2011; Cruz et al, Nat Neurosci Rev 2013) a, By FACS, MAP2 positive population were gated and further assessed by other markers such as Iba1 (microglia/macrophage) and GFAP (astrocyte) in brain cell samples isolated from C57Bl6 mice. These comparisons confirmed that the MAP2+ population did not contain any appreciable amounts of microglia or astrocyte, whereas another neuron marker (NeuN) was highly enriched. b, Similar findings were obtained using an alternative gating method to isolate neurons.
Extended Data Figure 10. Involvement of integrin-mediate src/syk mechanisms in astrocytic mitochondrial entry into neurons in vitro
a and b, Cultured rat cortical astrocytes were stimulated by cADPR (1 μM) for 24 hours. Intracellular mitochondria labeled by mitotracker dye was significantly increased in astrocytes stimulated with cADPR (1 μM) (n=7).**P<0.01 vs 0h. c, Some of mitochondria were found outside of cells. d, FACS analysis revealed that approximately 5×105 mitochondria were contained in 1mL of astrocyte-derived conditioned media (n=6). cADPR (1 μM) significantly increased the number of mitochondria in the media (n=6). e, Experimental schedule to quantify the mitochondrial entry into neurons following oxygen-glucose deprivation. Rat cortical neurons (1×105 cells/well) were prepared in 24-well culture plate. ACM or cADPR-ACM (each 1 mL) was co-incubated with neurons for 18 hours. Mitochondrial entry into neurons were calculated by mitochondrial intensity measured before and after washing cells with PBS. Phenol red free culture media were used to decrease back ground signal. Back ground signal was subtracted from fluorescent intensity obtained from each sample. f, Oxygen-glucose deprivation for 2 hours decreased approximately 50% of mitochondria in neurons after 18 h reoxygenation (n=4). g, All data are expressed as relative values, with total neuronal mitochondria after 2 h OGD/18 h reoxygenation being 100%. Mitochondrial entry into neurons was slightly higher in cADPR-ACM treatment (18%) compared to ACM treatment (11%), although there was no statistically significance (n=4). h, There was no difference in the percentage of mitochondrial entry between ACM treatment and cADPR-ACM treatment (n=4). i, cADPR-ACM treatment supported neuronal viability better than ACM treatment (n=4). **j,**Co-culture between rat cortical astrocytes in the upper chamber and rat cortical neurons in the lower chamber was performed for 18 hours following oxygen-glucose deprivation for 2 hours in neurons. Then, mitochondrial entry into neurons was measured. k, Immediately after oxygen-glucose deprivation, dynasore (5 μM), RGDS peptide (50 μg/ml), or MNS (1 μM) was initially added in neurons for 30 min, then astrocyte co-culture was performed for 18 hours. The data are expressed as relative values, with astrocytic extracellular mitochondria plus entered mitochondria into neurons being 100%. RGDS peptide and MNS significantly decreased mitochondrial entry into neurons, but dynasore did not inhibit the entry. l, MNS treatment significantly decreased astrocyte-mediated neuroprotection (n=4). m, Dynasore (5 μM), RGDS peptide (50 μg/ml), or MNS (1 μM) did not affect neuronal viability after 2 h oxygen-glucose deprivation (n=4). All values are mean +/− SEM. These data suggest that astrocyte into neuron mitochondrial particle entry may involve integrin-mediate src/syk mechanisms. However, we acknowledge that these pathways may be multifactorial and deeper analyses are warranted to dissect entry mechanisms under various physiologic and pathologic conditions.
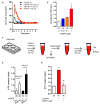
Fig. 1. Astrocytic CD38 and extracellular mitochondria
a, Transmission electron microscopy (TEM) of extracellular mitochondria in astrocyte-conditioned medium (ACM). Scale: 500 nm.b, Rat cortical astrocytes were labeled by Mitotracker Red CMXRos. FACS showed that 0.2 μm filter depleted extracellular mitochondria in ACM (mdACM). c–e, 0.2 μm filters reduced markers of extracellular mitochondrial function in ACM - **c,**extracellular ATP (n=4), d, membrane potential (n=4), e, oxygen consumption (n= 9 or 6).f, Western blot confirmed higher CD38 in rat cortical astrocytes compared to neurons. g, High and low levels of CD38 cyclase activity in astrocytes and neurons respectively (n= 8 or 5).h,. Experimental schematic for testing CRISPR/Cas9-mediated CD38 activation. i, Twenty four hours after transfection, CD38 cyclase activity was upregulated by CD38 activation plasmid (n=4).j, k, Extracellular ATP production (j) and oxygen consumption (k) were significantly increased by CD38 activation (n=5). l, FACS showed that extracellular mitochondria were increased by cADPR (1 μM) stimulation in astrocytes (n=3).m, cADPR (1 μM) increased extracellular mitochondria membrane potential at 24 hours (n=7). n, Oxygen consumption in extracellular mitochondria was increased by cADPR (n=4).o, cADPR did not cause astrocyte toxicity (n=4). All values are mean +/− SEM.
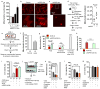
Fig. 2. Astrocytic extracellular mitochondria and neuroprotection
a, Experimental schematic to test neuroprotective effects of astrocyte conditioned media (ACM) or mitochondria-depleted astrocyte conditioned media (mdACM) against oxygen-glucose deprivation (OGD) in rat cortical neurons.b, ACM but not mdACM rescued ATP levels in damaged neurons (n=4). c, ACM but not mdACM recovered neuronal viability after OGD (n=4). d, Immunostaining confirmed that neuroprotective effect of ACM but not mdACM (n=4). Scale: 100 μm. e, No statistically significant neuroprotection with liposomal ATP (1–1000 nM) after OGD. f, Fluorescent microscopy suggests the presence of astrocyte mitochondria (labeled with Mitotracker Red CMXRos, 200 nM) within neurons. Scale: 100 μm. All values are mean +/− SEM.
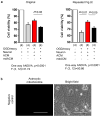
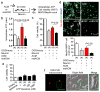
Fig. 3. Astrocytic mitochondria and neuroplasticity after ischemic stress
a, Confocal microscopy revealed that astrocytic mitochondria (red, Mitotracker Red CMXRos) may be transferred into neural soma (a) and axon (b), and some may fuse with neuronal mitochondria (c, green, Cell-light Mito-GFP).b, Experimental schematic for co-culture studies.c, Soma size was unchanged but astrocytic mitochondrial density in neuronal soma was significantly decreased when CD38 was suppressed in astrocytes (n=54 or 41 soma were counted). Scale: 20 μm.d, Quantification of dendrite elongation (MAP2 staining) (n=5 or 6). e, Male C57Bl6 mice were subjected to 60 min transient focal ischemia. Three days later, astrocyte mitochondria particles (1,000 particles/2μl, MitoTracker Red CMXRos) were infused into cerebral cortex. Confocal images showed transplanted astrocytic mitochondria (red) within peri-infarct neurons at 24 hrs. f, FVB/N-Tg (GFAPGFP)14Mes/J transgenic mice with fluorescently labeled astrocytes were subjected to 30 min transient focal ischemia. Immunohistochemistry at 24 hrs suggested that GFP (GFAP)-positive particles co-stained with mitochondrial TOM40 were present in MAP2-positive neurons in peri-infarct cortex. g, Western blot indicated that GFP-positive neurons upregulated cell survival-related proteins (phospho-Akt, Bcl-xl) but not apoptosis-related proteins (caspase 3, AIF) along with an increase of mitochondrial TOM40 (n=3). Isolated neurons expressed mature (neurofilament) but not neural stem cell markers (nestin) (Extended Data Fig. 7f). All values are mean +/− SEM.
Fig. 4. Effects of CD38 siRNA in focal cerebral ischemia
a, Male C57Bl6 mice were subjected to transient 60 min focal ischemia and control siRNA or CD38 siRNA was injected into lateral ventricles at 5 days post-stroke. Immunostaining showed that CD38 siRNA decreased HMGB1 astrocytes in peri-infarct cortex. b, Nissl staining showed no difference in infarct size (n=8 or 10). c, Immunostaining demonstrated that astrocytic CD38 was diminished by CD38 siRNA. **d,**Astrocytic CD38 suppression with siRNA reduced GFAP-positive mitochondria in CSF at 7 days (n=6). e, Neuronal mitochondria were decreased by CD38 siRNA (n= 8 or 5). f, CD38 siRNA attenuated peri-infarct GAP43 immunostaining. g, Western blot confirmed a reduction of peri-infarct GAP43 protein within CD38 siRNA-treated brains (n=5). h, i, Suppression of CD38 signaling worsened neurological outcomes in neuroscore (h) and grid walking test (i) (n=7 or 9).*P<0.05 vs day 3 control siRNA, #P<0.05 vs day 7 CD38 siRNA. j, CD38 suppression decreased oxygen consumption in CSF mitochondria (n=7 or 9). k, l, Mitochondrial function in CSF was negatively correlated with neurological outcomes. All values are mean +/− SEM. m, Schematic of CD38 regulation of mitochondria release/transfer hypothesis between astrocytes and neurons.
Comment in
- Mitochondrial Transfer from Astrocytes to Neurons following Ischemic Insult: Guilt by Association?
Berridge MV, Schneider RT, McConnell MJ. Berridge MV, et al. Cell Metab. 2016 Sep 13;24(3):376-378. doi: 10.1016/j.cmet.2016.08.023. Cell Metab. 2016. PMID: 27626198 - Astrocyte power fuels neurons during stroke.
Pluchino S, Peruzzotti-Jametti L, Frezza C. Pluchino S, et al. Swiss Med Wkly. 2016 Nov 10;146:w14374. doi: 10.4414/smw.2016.14374. eCollection 2016. Swiss Med Wkly. 2016. PMID: 27878792 No abstract available. - Transfer of mitochondria after stroke: a new hope for cardioprotection coming from the brain?
Fauconnier J. Fauconnier J. Cardiovasc Res. 2017 May 1;113(6):e10-e11. doi: 10.1093/cvr/cvx057. Cardiovasc Res. 2017. PMID: 28453738 No abstract available.
Similar articles
- TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri-infarct depolarizations in a stroke model.
Rakers C, Schmid M, Petzold GC. Rakers C, et al. Glia. 2017 Sep;65(9):1550-1561. doi: 10.1002/glia.23183. Epub 2017 Jun 22. Glia. 2017. PMID: 28639721 - CD38 positively regulates postnatal development of astrocytes cell-autonomously and oligodendrocytes non-cell-autonomously.
Hattori T, Kaji M, Ishii H, Jureepon R, Takarada-Iemata M, Minh Ta H, Manh Le T, Konno A, Hirai H, Shiraishi Y, Ozaki N, Yamamoto Y, Okamoto H, Yokoyama S, Higashida H, Kitao Y, Hori O. Hattori T, et al. Glia. 2017 Jun;65(6):974-989. doi: 10.1002/glia.23139. Epub 2017 Mar 13. Glia. 2017. PMID: 28295574 - Postnatal expression of CD38 in astrocytes regulates synapse formation and adult social memory.
Hattori T, Cherepanov SM, Sakaga R, Roboon J, Nguyen DT, Ishii H, Takarada-Iemata M, Nishiuchi T, Kannon T, Hosomichi K, Tajima A, Yamamoto Y, Okamoto H, Sugawara A, Higashida H, Hori O. Hattori T, et al. EMBO J. 2023 Aug 1;42(15):e111247. doi: 10.15252/embj.2022111247. Epub 2023 Jun 26. EMBO J. 2023. PMID: 37357972 Free PMC article. - Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection.
Bambrick L, Kristian T, Fiskum G. Bambrick L, et al. Neurochem Res. 2004 Mar;29(3):601-8. doi: 10.1023/b:nere.0000014830.06376.e6. Neurochem Res. 2004. PMID: 15038607 Review. - Pathological alterations of astrocytes in stroke-prone spontaneously hypertensive rats under ischemic conditions.
Yamagata K. Yamagata K. Neurochem Int. 2012 Jan;60(1):91-8. doi: 10.1016/j.neuint.2011.11.002. Epub 2011 Nov 15. Neurochem Int. 2012. PMID: 22100568 Review.
Cited by
- Molecular and cellular mechanisms of mitochondria transfer in models of central nervous system disease.
Nakano T, Irie K, Matsuo K, Mishima K, Nakamura Y. Nakano T, et al. J Cereb Blood Flow Metab. 2024 Nov 14:271678X241300223. doi: 10.1177/0271678X241300223. Online ahead of print. J Cereb Blood Flow Metab. 2024. PMID: 39539186 Free PMC article. Review. - Mechanisms Regulating Mitochondrial Transfer in Human Corneal Epithelial Cells.
Pal-Ghosh S, Karpinski BA, Datta-Majumdar H, Datta S, Dimri S, Hally J, Wehmeyer H, Stepp MA. Pal-Ghosh S, et al. Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):10. doi: 10.1167/iovs.65.13.10. Invest Ophthalmol Vis Sci. 2024. PMID: 39504055 Free PMC article. - Mitochondrial transfer balances cell redox, energy and metabolic homeostasis in the osteoarthritic chondrocyte preserving cartilage integrity.
Court AC, Vega-Letter AM, Parra-Crisóstomo E, Velarde F, García C, Ortloff A, Vernal R, Pradenas C, Luz-Crawford P, Khoury M, Figueroa FE. Court AC, et al. Theranostics. 2024 Oct 7;14(17):6471-6486. doi: 10.7150/thno.96723. eCollection 2024. Theranostics. 2024. PMID: 39479450 Free PMC article. - Role of damaged mitochondrial transfer in alpha-particle generator 212Pb radiation-induced bystander effect.
Yang M, Wang L, Qin S, Dai X, Li J, An L, Song L, Gao J, Han Z, Yu F. Yang M, et al. Theranostics. 2024 Oct 14;14(17):6768-6782. doi: 10.7150/thno.101922. eCollection 2024. Theranostics. 2024. PMID: 39479441 Free PMC article. - Mitochondrial Bioenergetics of Functional Wound Closure is Dependent on Macrophage-Keratinocyte Exosomal Crosstalk.
Sharma A, Srivastava R, Gnyawali SC, Bhasme P, Anthony AJ, Xuan Y, Trinidad JC, Sen CK, Clemmer DE, Roy S, Ghatak S. Sharma A, et al. ACS Nano. 2024 Nov 5;18(44):30405-30420. doi: 10.1021/acsnano.4c07610. Epub 2024 Oct 25. ACS Nano. 2024. PMID: 39453865 Free PMC article.
References
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. - PubMed
- Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103:162–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials