Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer - PubMed (original) (raw)
Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer
Yong Hyun Park et al. Sci Rep. 2016.
Erratum in
- Author Correction: Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer.
Park YH, Shin HW, Jung AR, Kwon OS, Choi YJ, Park J, Lee JY. Park YH, et al. Sci Rep. 2019 Apr 15;9(1):6051. doi: 10.1038/s41598-019-41385-w. Sci Rep. 2019. PMID: 30988318 Free PMC article.
Abstract
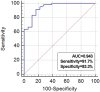
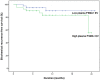
Extracellular vesicles (EVs) may play an important role in cancer development and progression. We aimed to investigate the prognostic potential of prostate-specific EVs in prostate cancer (PCa) patients. Plasma and prostate tissue were collected from patients who underwent surgery for PCa (n = 82) or benign prostatic hyperplasia (BPH, n = 28). To analyze the quantity of EVs in prostate, we performed transmission electron microscopy (TEM), immuno-TEM with CD63 and prostate-specific membrane antigen (PSMA), and immunofluorescence staining. After EV isolation from plasma, CD63 and PSMA concentration was measured using ELISA kits. PSMA-positive areas in prostate differed in patients with BPH, and low-, intermediate-, and high-risk PCa (2.4, 8.2, 17.5, 26.5%, p < 0.001). Plasma PSMA-positive EV concentration differed in patients with BPH, and low-, intermediate-, and high-risk PCa (21.9, 43.4, 49.2, 59.9 ng/mL, p < 0.001), and ROC curve analysis indicated that plasma PSMA-positive EV concentration differentiated PCa from BPH (AUC 0.943). Patients with lower plasma PSMA-positive EV concentration had greater prostate volume (50.2 vs. 33.4 cc, p < 0.001) and lower pathologic Gleason score (p = 0.025). During the median follow-up of 18 months, patients with lower plasma PSMA-positive EV concentration tended to have a lower risk of biochemical failure than those with higher levels of prostate-specific EVs (p = 0.085).
Figures
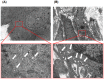
Figure 1. Representative transmission electron miscroscopy (TEM) images of extracellular vesicles (EVs) in prostate tissue.
Vesicles 30–100 nm in diameter were observed by TEM. (A) Human benign prostatic hyperplasia (BPH) cells produce several microvesicles. The lower panel shows a magnified region of (A). The EVs appear as white dots (indicated by an arrow). (B) Human prostate cancer cells shed more microvesicles compared to BPH cells. The lower panel shows a magnified region of (B) Bars in low-magnification images, 1 μm. Bars in high-magnification images, 200 nm.
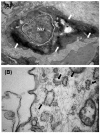
Figure 2
Representative TEM images of (A) immunoperoxidase/diaminobenzidine methods and (B) immunogold enhancement showing ultrastructural localization of PSMA. Bar in (A) 1 μm. Bar in (B) 10 nm.
Figure 3
Representative images of immunofluorescence staining for CD63 and PSMA in patients with (A) benign prostatic hyperplasia and (B) prostate cancer. (C) Quantification of PSMA-positive areas in prostatic tissue (p < 0.001).
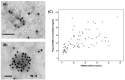
Figure 4
Representative images of TEM with immunogold enhancement with anti-CD63 (A) and PSMA (B) antibodies. (C) Correlation between the plasma PSMA-positive EV concentration and PSMA-positive areas in prostatic tissue (Spearman’s rho correlation coefficient = 0.672, p < 0.001).
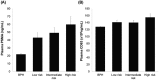
Figure 5
Quantification of the concentration of (A) plasma PSMA-positive EV (p < 0.001) and (B) plasma CD63-positive EV (p = 0.067).
Figure 6. Receiver operating characteristic curve analysis using plasma PSMA-positive EV concentration for discrimination of prostate cancer from benign prostatic hyperplasia.
Figure 7. Biochemical recurrence free-survival according to plasma PSMA-positive EV concentration (p = 0.085).
Similar articles
- Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma.
Padda RS, Deng FK, Brett SI, Biggs CN, Durfee PN, Brinker CJ, Williams KC, Leong HS. Padda RS, et al. Prostate. 2019 May;79(6):592-603. doi: 10.1002/pros.23764. Epub 2019 Jan 24. Prostate. 2019. PMID: 30680751 - Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry.
Biggs CN, Siddiqui KM, Al-Zahrani AA, Pardhan S, Brett SI, Guo QQ, Yang J, Wolf P, Power NE, Durfee PN, MacMillan CD, Townson JL, Brinker JC, Fleshner NE, Izawa JI, Chambers AF, Chin JL, Leong HS. Biggs CN, et al. Oncotarget. 2016 Feb 23;7(8):8839-49. doi: 10.18632/oncotarget.6983. Oncotarget. 2016. PMID: 26814433 Free PMC article. - Metabolic profiling in tissues and urine of patients with prostatic lesions and the diagnostic value of urine extracellular vesicles metabolites in prostate cancer.
Ding T, He W, Yan H, Wei Z, Zeng X, Hao X. Ding T, et al. Clin Chim Acta. 2024 Mar 15;556:117845. doi: 10.1016/j.cca.2024.117845. Epub 2024 Feb 23. Clin Chim Acta. 2024. PMID: 38403146 - Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis.
Pang B, Zhu Y, Ni J, Thompson J, Malouf D, Bucci J, Graham P, Li Y. Pang B, et al. Theranostics. 2020 Jan 16;10(5):2309-2326. doi: 10.7150/thno.39486. eCollection 2020. Theranostics. 2020. PMID: 32089744 Free PMC article. Review. - Extracellular Vesicle Proteome in Prostate Cancer: A Comparative Analysis of Mass Spectrometry Studies.
Bernardino RMM, Leão R, Henrique R, Pinheiro LC, Kumar P, Suravajhala P, Beck HC, Carvalho AS, Matthiesen R. Bernardino RMM, et al. Int J Mol Sci. 2021 Dec 19;22(24):13605. doi: 10.3390/ijms222413605. Int J Mol Sci. 2021. PMID: 34948404 Free PMC article. Review.
Cited by
- Prostate cancer-derived small extracellular vesicle proteins: the hope in diagnosis, prognosis, and therapeutics.
Chen H, Pang B, Zhou C, Han M, Gong J, Li Y, Jiang J. Chen H, et al. J Nanobiotechnology. 2023 Dec 14;21(1):480. doi: 10.1186/s12951-023-02219-0. J Nanobiotechnology. 2023. PMID: 38093355 Free PMC article. Review. - Clinical Significance of Extracellular Vesicles in Prostate and Renal Cancer.
Chen TY, Mihalopoulos M, Zuluaga L, Rich J, Ganta T, Mehrazin R, Tsao CK, Tewari A, Gonzalez-Kozlova E, Badani K, Dogra N, Kyprianou N. Chen TY, et al. Int J Mol Sci. 2023 Sep 28;24(19):14713. doi: 10.3390/ijms241914713. Int J Mol Sci. 2023. PMID: 37834162 Free PMC article. Review. - Isolation and Characterization of Small Extracellular Vesicles from Porcine Blood Plasma, Cerebrospinal Fluid, and Seminal Plasma.
Skalnikova HK, Bohuslavova B, Turnovcova K, Juhasova J, Juhas S, Rodinova M, Vodicka P. Skalnikova HK, et al. Proteomes. 2019 Apr 25;7(2):17. doi: 10.3390/proteomes7020017. Proteomes. 2019. PMID: 31027284 Free PMC article. - Expression of CD13 and CD26 on extracellular vesicles in canine seminal plasma: preliminary results.
Troisi A, Schrank M, Bellezza I, Fallarino F, Pastore S, Verstegen JP, Pieramati C, Di Michele A, Talesa VN, Martìnez Barbitta M, Orlandi R, Polisca A. Troisi A, et al. Vet Res Commun. 2024 Feb;48(1):357-366. doi: 10.1007/s11259-023-10202-1. Epub 2023 Sep 14. Vet Res Commun. 2024. PMID: 37707657 Free PMC article. - Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids.
Logozzi M, Di Raimo R, Mizzoni D, Fais S. Logozzi M, et al. Methods Enzymol. 2020;645:155-180. doi: 10.1016/bs.mie.2020.06.011. Epub 2020 Jul 9. Methods Enzymol. 2020. PMID: 33565970 Free PMC article. Review.
References
- Stamey T. A. et al. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? The Journal of urology 172, 1297–1301 (2004). - PubMed
- Trams E. G., Lauter C. J., Salem N. Jr. & Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta 645, 63–70 (1981). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous