Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study - PubMed (original) (raw)
Randomized Controlled Trial
. 2017 Jan 15;595(2):541-555.
doi: 10.1113/JP272613. Epub 2016 Sep 18.
Sara V Gomand 2 3, Lise Deroover 1 2, Tom Preston 4, Karen Vermeulen 5, Vicky De Preter 1 6, Henrike M Hamer 1 2, Guy Van den Mooter 7, Luc De Vuyst 8, Christophe M Courtin 2 3, Pieter Annaert 7, Jan A Delcour 2 3, Kristin A Verbeke 1 2
Affiliations
- PMID: 27510655
- PMCID: PMC5233652
- DOI: 10.1113/JP272613
Randomized Controlled Trial
Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study
Eef Boets et al. J Physiol. 2017.
Abstract
Key points: The short-chain fatty acids (SCFAs) are bacterial metabolites produced during the colonic fermentation of undigested carbohydrates, such as dietary fibre and prebiotics, and can mediate the interaction between the diet, the microbiota and the host. We quantified the fraction of colonic administered SCFAs that could be recovered in the systemic circulation, the fraction that was excreted via the breath and urine, and the fraction that was used as a precursor for glucose, cholesterol and fatty acids. This information is essential for understanding the molecular mechanisms by which SCFAs beneficially affect physiological functions such as glucose and lipid metabolism and immune function.
Abstract: The short-chain fatty acids (SCFAs), acetate, propionate and butyrate, are bacterial metabolites that mediate the interaction between the diet, the microbiota and the host. In the present study, the systemic availability of SCFAs and their incorporation into biologically relevant molecules was quantified. Known amounts of 13 C-labelled acetate, propionate and butyrate were introduced in the colon of 12 healthy subjects using colon delivery capsules and plasma levels of 13 C-SCFAs 13 C-glucose, 13 C-cholesterol and 13 C-fatty acids were measured. The butyrate-producing capacity of the intestinal microbiota was also quantified. Systemic availability of colonic-administered acetate, propionate and butyrate was 36%, 9% and 2%, respectively. Conversion of acetate into butyrate (24%) was the most prevalent interconversion by the colonic microbiota and was not related to the butyrate-producing capacity in the faecal samples. Less than 1% of administered acetate was incorporated into cholesterol and <15% in fatty acids. On average, 6% of colonic propionate was incorporated into glucose. The SCFAs were mainly excreted via the lungs after oxidation to 13 CO2 , whereas less than 0.05% of the SCFAs were excreted into urine. These results will allow future evaluation and quantification of SCFA production from 13 C-labelled fibres in the human colon by measurement of 13 C-labelled SCFA concentrations in blood.
Keywords: colonic fermentation; metabolism; short-chain fatty acids; stable isotopes; systemic exposure.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
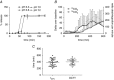
Figure 1. In vitro and in vivo evaluation of the performance of the colon delivery capsules
A, in vitro dissolution profile of colon delivery capsules at different pH (n = 2). B, 14CO2 and 13CO2 appearance in breath after administration of 13C‐acetate, 13C‐propionate and 13C‐butyrate colon delivery capsules in 12 subjects. The simultaneous rise in the breath of 14CO2 and 13CO2 indicates a correct delivery of 13C‐acetate in the colon. C, comparison of the time of release of the 13C‐SCFAs from the capsules (indicated by the time point at which 20% of the cumulative amount recovered was obtained, t20%) and arrival of the test meal in the colon (indicated by the orocecal transit time, OCTT); n = 36, except for OCTT (n = 30). Results are expressed as the mean ± SD.
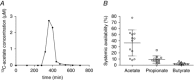
Figure 2. Calculation of the systemic availability of colonic‐administered 13C‐SCFAs
A, Typical graph depicting 13C‐acetate concentrations in plasma vs. time. B, systemic availability results of acetate, propionate and butyrate for all 12 subjects. Results are expressed as the mean ± SD.
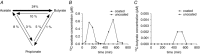
Figure 3. Cross‐feeding of SCFAs
A, overview and quantitative indication of the interconversions between acetate, propionate and butyrate (n = 12). B, appearance of 13C‐acetate in plasma after administration of a coated and uncoated capsule filled with 13C‐acetate. Without coating, 13C‐acetate was released in the proximal intestine and appears at an earlier time in plasma compared to a coated capsule. C, appearance of 13C‐butyrate in plasma after administration of a coated and uncoated capsule filled with 13C‐acetate. 13C‐butyrate is only formed when 13C‐acetate is properly released in the colon.
Figure 4. Butyrate‐producing capacity
A, gene copy numbers of butyrate‐producing colon bacteria and butyrate‐producing colon enzymes in faecal samples (n = 11). B_–_C, correlation between acetate into butyrate conversion and enzymes involved in butyrate synthesis (n = 11). D_–_E, correlation between acetate into butyrate conversion and the most abundant butyrate‐producing bacteria (n = 11).
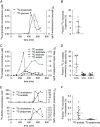
Figure 5. Assimilation of 13C‐SCFAs in biologically relevant molecules
A, typical example that shows the appearance of 13C‐propionate followed by 13C‐glucose in plasma after colonic administration of 13C‐propionate. B, fraction of administered 13C‐propionate recovered in glucose (n = 12). C, typical example that shows the appearance of 13C‐acetate followed by 13C‐palmitate, 13C‐stearate and 13C‐oleate in plasma after colonic administration of 13C‐acetate. D, fraction of administered 13C‐acetate recovered in palmitate (C16), stearate (C18) and oleate (C18:1) (n = 12). E, typical examples that show the appearance of 13C‐acetate and 13C‐propionate followed by 13C‐cholesterol in plasma after colonic administration of 13C‐acetate and 13C‐propionate. F, fraction of administered 13C‐acetate and 13C‐propionate recovered in cholesterol (n = 12). Results are expressed as the mean ± SD.
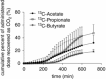
Figure 6. Recovery of 13C‐acetate, 13C‐propionate and 13C‐butyrate in the breath as 13CO2
Results are expressed as the mean ± SD (n = 12).
Comment in
- Progress in the biology and analysis of short chain fatty acids.
Vonk RJ, Reckman G. Vonk RJ, et al. J Physiol. 2017 Jan 15;595(2):419-420. doi: 10.1113/JP273260. J Physiol. 2017. PMID: 28083944 Free PMC article. No abstract available.
Similar articles
- Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin.
Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K. Boets E, et al. Nutrients. 2015 Oct 28;7(11):8916-29. doi: 10.3390/nu7115440. Nutrients. 2015. PMID: 26516911 Free PMC article. - Colonic health: fermentation and short chain fatty acids.
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Wong JM, et al. J Clin Gastroenterol. 2006 Mar;40(3):235-43. doi: 10.1097/00004836-200603000-00015. J Clin Gastroenterol. 2006. PMID: 16633129 Review. - Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids.
den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, Reijngoud DJ. den Besten G, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Dec;305(12):G900-10. doi: 10.1152/ajpgi.00265.2013. Epub 2013 Oct 17. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 24136789 - Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.
van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. van de Wouw M, et al. J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article. - Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease.
Mortensen PB, Clausen MR. Mortensen PB, et al. Scand J Gastroenterol Suppl. 1996;216:132-48. doi: 10.3109/00365529609094568. Scand J Gastroenterol Suppl. 1996. PMID: 8726286 Review.
Cited by
- Microbiota-derived short chain fatty acids in pediatric health and diseases: from gut development to neuroprotection.
Hsu CY, Khachatryan LG, Younis NK, Mustafa MA, Ahmad N, Athab ZH, Polyanskaya AV, Kasanave EV, Mirzaei R, Karampoor S. Hsu CY, et al. Front Microbiol. 2024 Oct 8;15:1456793. doi: 10.3389/fmicb.2024.1456793. eCollection 2024. Front Microbiol. 2024. PMID: 39439941 Free PMC article. Review. - Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities.
Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Al-Qadami GH, et al. Microorganisms. 2022 Oct 17;10(10):2048. doi: 10.3390/microorganisms10102048. Microorganisms. 2022. PMID: 36296324 Free PMC article. Review. - The effects of SCFAs on glycemic control in humans: a systematic review and meta-analysis.
Cherta-Murillo A, Pugh JE, Alaraj-Alshehhi S, Hajjar D, Chambers ES, Frost GS. Cherta-Murillo A, et al. Am J Clin Nutr. 2022 Aug 4;116(2):335-361. doi: 10.1093/ajcn/nqac085. Am J Clin Nutr. 2022. PMID: 35388874 Free PMC article. - Effects of Whole Milk Supplementation on Gut Microbiota and Cardiometabolic Biomarkers in Subjects with and without Lactose Malabsorption.
Li X, Yin J, Zhu Y, Wang X, Hu X, Bao W, Huang Y, Chen L, Chen S, Yang W, Shan Z, Liu L. Li X, et al. Nutrients. 2018 Oct 2;10(10):1403. doi: 10.3390/nu10101403. Nutrients. 2018. PMID: 30279333 Free PMC article. Clinical Trial. - Targeting gut dysbiosis against inflammation and impaired autophagy in Duchenne muscular dystrophy.
Kalkan H, Pagano E, Paris D, Panza E, Cuozzo M, Moriello C, Piscitelli F, Abolghasemi A, Gazzerro E, Silvestri C, Capasso R, Motta A, Russo R, Di Marzo V, Iannotti FA. Kalkan H, et al. EMBO Mol Med. 2023 Mar 8;15(3):e16225. doi: 10.15252/emmm.202216225. Epub 2023 Jan 3. EMBO Mol Med. 2023. PMID: 36594243 Free PMC article.
References
- Annison G, Illman RJ & Topping DL (2003). Acetylated, propionylated or butyrylated starches raise large bowel short‐chain fatty acids preferentially when fed to rats. J Nutr 133, 3523–3528. - PubMed
- Bergman EN (1990). Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70, 567–590. - PubMed
- Bjorntorp P & Sjostrom L (1978). Carbohydrate storage in man: speculations and some quantitative considerations. Metab Clin Exp 27, 1853–1865. - PubMed
- Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA & Dejong CH (2009). Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr 28, 657–661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources