Polyamines Confer Salt Tolerance in Mung Bean (Vigna radiata L.) by Reducing Sodium Uptake, Improving Nutrient Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification Systems - PubMed (original) (raw)
Polyamines Confer Salt Tolerance in Mung Bean (Vigna radiata L.) by Reducing Sodium Uptake, Improving Nutrient Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification Systems
Kamrun Nahar et al. Front Plant Sci. 2016.
Abstract
The physiological roles of PAs (putrescine, spermidine, and spermine) were investigated for their ability to confer salt tolerance (200 mM NaCl, 48 h) in mung bean seedlings (Vigna radiata L. cv. BARI Mung-2). Salt stress resulted in Na toxicity, decreased K, Ca, Mg, and Zn contents in roots and shoots, and disrupted antioxidant defense system which caused oxidative damage as indicated by increased lipid peroxidation, H2O2 content, [Formula: see text] generation rate, and lipoxygenase activity. Salinity-induced methylglyoxal (MG) toxicity was also clearly evident. Salinity decreased leaf chlorophyll (chl) and relative water content (RWC). Supplementation of salt affected seedlings with exogenous PAs enhanced the contents of glutathione and ascorbate, increased activities of antioxidant enzymes (dehydroascorbate reductase, glutathione reductase, catalase, and glutathione peroxidase) and glyoxalase enzyme (glyoxalase II), which reduced salt-induced oxidative stress and MG toxicity, respectively. Exogenous PAs reduced cellular Na content and maintained nutrient homeostasis and modulated endogenous PAs levels in salt affected mung bean seedlings. The overall salt tolerance was reflected through improved tissue water and chl content, and better seedling growth.
Keywords: ROS signaling; abiotic stress; methylglyoxal; oxidative damage; polyamine; salinity.
Figures
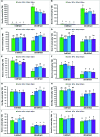
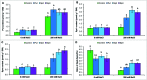
FIGURE 1
Effect of exogenous polyamines on Na uptake and nutrient homeostasis in mung bean seedlings under salt stress (NaCl, 200 mM). Root Na (A), shoot Na (B), root K (C), and shoot K (D) root Ca (E), shoot Ca (F), root Mg (G), shoot Mg (H), root Zn (I), and shoot Zn (J) contents. Here, Put, Spd, and Spm indicate putrescine (0.2 mM), spermidine (0.2 mM), and spermine (0.2 mM), respectively. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P ≤ 0.05 applying Tukey’s HSD test.
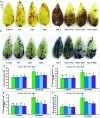
FIGURE 2
Histochemical detection of ROS in leaves, content of ROS, LOX activity, and membrane lipid peroxidation in salt (NaCl, 200 mM) affected mung bean seedlings. DAB staining (A) of H2O2 and NBT staining (B) of O2•- in leaves, H2O2 content (C), O2•- generation rate (D), LOX activity (E), and MDA content (F). Here, Put, Spd, and Spm indicate putrescine (0.2 mM), spermidine (0.2 mM), and spermine (0.2 mM), respectively. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P ≤ 0.05 applying Tukey’s HSD test.
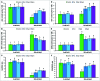
FIGURE 3
Effect of exogenous polyamines on non-enzymatic antioxidants in mung bean seedlings under salt (NaCl, 200 mM) stress. Ascorbate (AsA) content (A), DHA content (B), AsA/DHA ratio (C), GSH content (D), GSSG content (E), and GSH/GSSG ratio (F). Here, Put, Spd, and Spm indicate putrescine (0.2 mM), spermidine (0.2 mM), and spermine (0.2 mM), respectively. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P ≤ 0.05 applying Tukey’s HSD test.
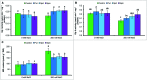
FIGURE 4
Effect of exogenous polyamines on glyoxalase enzymes and in reducing MG toxicity in mung bean seedlings subjected to salt (NaCl, 200 mM) stress. Activity of Gly I (A), activity of Gly II (B), and MG content (C). Here, Put, Spd, and Spm indicate putrescine (0.2 mM), spermidine (0.2 mM), and spermine (0.2 mM), respectively. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P ≤ 0.05 applying Tukey’s HSD test.
FIGURE 5
Endogenous levels of free polyamines in salt (NaCl, 200 mM) affected mung bean seedlings induced by treatment with exogenous polyamines. Endogenous Put (A), Spd (B), and Spm (C) contents and the ratio of (Spd+Spm)/Put (D). Here, Put, Spd, and Spm indicate exogenous putrescine (0.2 mM), spermidine (0.2 mM), and spermine (0.2 mM), respectively. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P ≤ 0.05, applying Tukey’s HSD test.
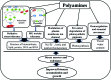
FIGURE 6
Mechanism of PAs-induced salt stress tolerance. In glyoxalase system, HA indicates hemithioacetal and SLG indicates S-
D
-lactoylglutathione.
Similar articles
- Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings.
Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Nahar K, et al. Environ Sci Pollut Res Int. 2016 Nov;23(21):21206-21218. doi: 10.1007/s11356-016-7295-8. Epub 2016 Aug 4. Environ Sci Pollut Res Int. 2016. PMID: 27491421 - Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems.
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Nahar K, et al. Ecotoxicol Environ Saf. 2016 Apr;126:245-255. doi: 10.1016/j.ecoenv.2015.12.026. Epub 2016 Jan 11. Ecotoxicol Environ Saf. 2016. PMID: 26773834 - Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems.
Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Nahar K, et al. AoB Plants. 2015 Jul 1;7:plv069. doi: 10.1093/aobpla/plv069. AoB Plants. 2015. PMID: 26134121 Free PMC article. - Exogenous Spermidine Alleviates Low Temperature Injury in Mung Bean (Vigna radiata L.) Seedlings by Modulating Ascorbate-Glutathione and Glyoxalase Pathway.
Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Nahar K, et al. Int J Mol Sci. 2015 Dec 17;16(12):30117-32. doi: 10.3390/ijms161226220. Int J Mol Sci. 2015. PMID: 26694373 Free PMC article. - Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems.
Nahar K, Hasanuzzaman M, Suzuki T, Fujita M. Nahar K, et al. Ecotoxicology. 2017 Jan;26(1):58-73. doi: 10.1007/s10646-016-1740-9. Epub 2016 Nov 7. Ecotoxicology. 2017. PMID: 27819117
Cited by
- Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation.
Ahanger MA, Qin C, Begum N, Maodong Q, Dong XX, El-Esawi M, El-Sheikh MA, Alatar AA, Zhang L. Ahanger MA, et al. BMC Plant Biol. 2019 Nov 8;19(1):479. doi: 10.1186/s12870-019-2085-3. BMC Plant Biol. 2019. PMID: 31703619 Free PMC article. - Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses.
Chen D, Shao Q, Yin L, Younis A, Zheng B. Chen D, et al. Front Plant Sci. 2019 Jan 10;9:1945. doi: 10.3389/fpls.2018.01945. eCollection 2018. Front Plant Sci. 2019. PMID: 30687350 Free PMC article. Review. - Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity.
Hasanuzzaman M, Raihan MRH, Masud AAC, Rahman K, Nowroz F, Rahman M, Nahar K, Fujita M. Hasanuzzaman M, et al. Int J Mol Sci. 2021 Aug 28;22(17):9326. doi: 10.3390/ijms22179326. Int J Mol Sci. 2021. PMID: 34502233 Free PMC article. Review. - Spermine reduces the harmful effects of salt stress in Tropaeolum majus.
da Silva TI, Dias MG, de Araújo NO, de Sousa Santos MN, Cruz RRP, Dias TJ, Ribeiro WS, Grossi JAS, Barbosa JG. da Silva TI, et al. Physiol Mol Biol Plants. 2022 Mar;28(3):687-696. doi: 10.1007/s12298-022-01165-9. Epub 2022 Mar 25. Physiol Mol Biol Plants. 2022. PMID: 35465202 Free PMC article. - Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants.
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Hasanuzzaman M, et al. Int J Mol Sci. 2017 Jan 20;18(1):200. doi: 10.3390/ijms18010200. Int J Mol Sci. 2017. PMID: 28117669 Free PMC article. Review.
References
- Addinsoft (2015). XLSTAT V. 2015.1.01: Data Analysis and Statistics Software for Microsoft Excel. Paris: Addinsoft.
- Barrs H. D., Weatherley P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 15 413–428. 10.1071/BI9620413 - DOI
- Bates L. S., Waldren R. P., Teari D. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39 205–207. 10.1007/BF00018060 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources