Are aberrant phase transitions a driver of cellular aging? - PubMed (original) (raw)
Review
Are aberrant phase transitions a driver of cellular aging?
Simon Alberti et al. Bioessays. 2016 Oct.
Abstract
Why do cells age? Recent advances show that the cytoplasm is organized into many membrane-less compartments via a process known as phase separation, which ensures spatiotemporal control over diffusion-limited biochemical reactions. Although phase separation is a powerful mechanism to organize biochemical reactions, it comes with the trade-off that it is extremely sensitive to changes in physical-chemical parameters, such as protein concentration, pH, or cellular energy levels. Here, we highlight recent findings showing that age-related neurodegenerative diseases are linked to aberrant phase transitions in neurons. We discuss how these aberrant phase transitions could be tied to a failure to maintain physiological physical-chemical conditions. We generalize this idea to suggest that the process of cellular aging involves a progressive loss of the organization of phase-separated compartments in the cytoplasm.
Keywords: aging; amyotrophic lateral sclerosis; chaperone; intrinsically disordered protein; mitochondria; neurodegeneration; phase separation; protein aggregation; protein quality control.
© 2016 The Authors BioEssays Published by WILEY Periodicals, Inc.
Figures
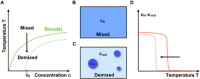
Figure 1
Sensitivity of phase separation to changing environmental conditions. A: Phase diagram of a binary mixture as a function of temperature T and concentration c. B and C: Upon decreasing the temperature an initially mixed state of mean concentration c 0 demixes, often leading to the formation of liquid droplets. D: The concentration of droplets inside minus outside, c in−c out, exhibits a jump‐like response when crossing the binodal line in A). Reverting the temperature change or weakly perturbing for example the pH or the salt concentration (dashed line in A and D) can dissolve the drops again.
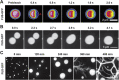
Figure 2
Dynamic behavior of FUS liquid droplets and droplet aging. A: Recovery of fluorescence intensity of an in vitro formed FUS–GFP droplet after half‐bleach. The site of photobleach is marked by a red arrow. B: Montage of two in vitro reconstituted FUS–GFP droplets fusing under shear flow. C: Aging of in vitro reconstituted liquid droplets into fibrous aggregates.
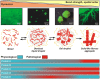
Figure 3
Continuum model of phase separation by intrinsically disordered proteins. An intrinsically disordered protein (shown in red) undergoes a phase transition into a liquid droplet by liquid–liquid demixing. The droplet “ages” with time and adopts different structures and material properties (gel, solid‐like fibrous aggregates). The images on the top show purified FUS protein forming liquid droplets, a gel, and fibrous aggregates 21. Bottom: The hypothetical proteins A–E span the whole range of the continuum. Pathological forms of these proteins are highlighted in red.
Similar articles
- Phase Separation: Linking Cellular Compartmentalization to Disease.
Aguzzi A, Altmeyer M. Aguzzi A, et al. Trends Cell Biol. 2016 Jul;26(7):547-558. doi: 10.1016/j.tcb.2016.03.004. Epub 2016 Apr 1. Trends Cell Biol. 2016. PMID: 27051975 Review. - Liquid-liquid phase separation in biology.
Hyman AA, Weber CA, Jülicher F. Hyman AA, et al. Annu Rev Cell Dev Biol. 2014;30:39-58. doi: 10.1146/annurev-cellbio-100913-013325. Annu Rev Cell Dev Biol. 2014. PMID: 25288112 Review. - Matter over mind: Liquid phase separation and neurodegeneration.
Elbaum-Garfinkle S. Elbaum-Garfinkle S. J Biol Chem. 2019 May 3;294(18):7160-7168. doi: 10.1074/jbc.REV118.001188. Epub 2019 Mar 26. J Biol Chem. 2019. PMID: 30914480 Free PMC article. Review. - A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation.
Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S. Patel A, et al. Cell. 2015 Aug 27;162(5):1066-77. doi: 10.1016/j.cell.2015.07.047. Cell. 2015. PMID: 26317470 - Bridging biophysics and neurology: aberrant phase transitions in neurodegenerative disease.
Nedelsky NB, Taylor JP. Nedelsky NB, et al. Nat Rev Neurol. 2019 May;15(5):272-286. doi: 10.1038/s41582-019-0157-5. Nat Rev Neurol. 2019. PMID: 30890779 Review.
Cited by
- Coupling Bulk Phase Separation of Disordered Proteins to Membrane Domain Formation in Molecular Simulations on a Bespoke Compute Fabric.
Shillcock JC, Thomas DB, Beaumont JR, Bragg GM, Vousden ML, Brown AD. Shillcock JC, et al. Membranes (Basel). 2021 Dec 23;12(1):17. doi: 10.3390/membranes12010017. Membranes (Basel). 2021. PMID: 35054543 Free PMC article. - Correlative all-optical quantification of mass density and mechanics of subcellular compartments with fluorescence specificity.
Schlüßler R, Kim K, Nötzel M, Taubenberger A, Abuhattum S, Beck T, Müller P, Maharana S, Cojoc G, Girardo S, Hermann A, Alberti S, Guck J. Schlüßler R, et al. Elife. 2022 Jan 10;11:e68490. doi: 10.7554/eLife.68490. Elife. 2022. PMID: 35001870 Free PMC article. - Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates.
Liu XM, Ma L, Schekman R. Liu XM, et al. Elife. 2021 Nov 12;10:e71982. doi: 10.7554/eLife.71982. Elife. 2021. PMID: 34766549 Free PMC article. - Molecular Crowding Tunes Material States of Ribonucleoprotein Condensates.
Kaur T, Alshareedah I, Wang W, Ngo J, Moosa MM, Banerjee PR. Kaur T, et al. Biomolecules. 2019 Feb 19;9(2):71. doi: 10.3390/biom9020071. Biomolecules. 2019. PMID: 30791483 Free PMC article. - Aberrant Compartment Formation by HSPB2 Mislocalizes Lamin A and Compromises Nuclear Integrity and Function.
Morelli FF, Verbeek DS, Bertacchini J, Vinet J, Mediani L, Marmiroli S, Cenacchi G, Nasi M, De Biasi S, Brunsting JF, Lammerding J, Pegoraro E, Angelini C, Tupler R, Alberti S, Carra S. Morelli FF, et al. Cell Rep. 2017 Aug 29;20(9):2100-2115. doi: 10.1016/j.celrep.2017.08.018. Cell Rep. 2017. PMID: 28854361 Free PMC article.
References
- Gems D, Partridge L. 2013. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75: 621–44. - PubMed
- Kirkwood TBL. 2005. Understanding the odd science of aging. Cell 120: 437–47. - PubMed
- Rattan SIS. 2008. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem 389: 267–72. - PubMed
- Burté F, Carelli V, Chinnery PF, Yu‐Wai‐Man P. 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol 11: 11–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical