Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol - PubMed (original) (raw)
Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol
Junqiang Zhang et al. Sci Rep. 2016.
Abstract
Resveratrol is a polyphenol derivatives which exhibits a pro-apoptotic effect in a variety of human cancers by triggering mitochondria apoptosis pathway and autophagy. However, there are scarcely reports on its apoptosis-promoting effect in abnormal proliferation fibroblast-like synoviocytes (FLSs). In this study, we investigated the underlying mechanism and apoptosis-inducing effects of resveratrol on the abnormal proliferation of FLSs in adjuvant-arthritis (AA) rats. Since using resveratrol for 12 days resulted in a significant decreasing the swelling degree of the paw, reducing malondialdehyde (MDA) content and enhancing superoxide dismutase (SOD) activity, antioxidant capacity, glutathione peroxidase and glutathione reductase ratio in AA rats. Moreover, we found that 5 μMH2O2 could increase cells viability, Beclin1, LC3A/B, MnSOD, SIRT3 protein expression in FLSs. But, resveratrol could reverse these effects by changing mitochondrial membrane potential (Δψm) to promote mitochondrial reactive oxygen species (mtROS) generation in 5 μMH2O2-treatment FLSs. These results suggest that oxidative stress existed in AA rats. Resveratrol could suppress oxidative stress in AA rats and increase mtROS production by reducing autophagy protein Beclin1, LC3A/B and oxidative stress protein MnSOD to promoted the apoptosis of FLSs. Thus, targeting of mtROS may be a crucial mechanism of resveratrol confers patients with rheumatoid arthritis.
Figures
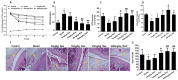
Figure 1. Arthritis induction in SD rats.
(A) Representative images show the effect on paw edema in rats with FCA-induced arthritis. (B) the nose and the double fore legs inflammation secondary injury in FCA-induced SD rats. (C) The severity of FCA-induced arthritis in SD rats. (D) The swelling degree of the paw in SD rats with FCA-induced arthritis, as determined by toe swelling apparatus calculated at the indicated time points. Values are the means ± SD of at least three independent experiments. *P < 0.05; **P < 0.01 versus control. n = 15/group.
Figure 2. Resveratrol reduces oxidative injury in AA rats.
after injecting FCA 20 days, treated with 5 mg/kg, 15 mg/kg, 45 mg/kg resveratrol and 200 mg/kg N-acetyl-L-cysteine (NAC) for 12 days by continuous intragastric administration. (A) The swelling degree of the paw in SD rats after intragastric administration. (B) Lipoperoxide levels in the serum from six groups rats. (C) SOD activity in six groups rats serum (D) Antioxidant capacity in six groups rats serum (E) The ratio of glutathione peroxidase and glutathione reductase in six groups rats serum. (F) HE staining of knee joint in AA rats after administration resveratrol. Data represent the means, aP < 0.05, bP < 0.01, cP < 0.01 versus model. Values are the means ± SD of at least three independent experiments. *P < 0.05 versus control; #P < 0.05, ##P < 0.01 versus model. n = 10/group.
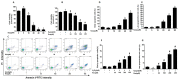
Figure 3. Resveratrol inhibited H2O2-treatment FLSs proliferation.
(A,B) Cells were treated with different concentrations (0, 5, 10,20, 40 and 80 μM) H2O2 for 12 h and cells were pretreated for 24 h with various concentrations of Res (0, 50,100,200 and 400 μM) before treatment with 5 μMH2O2 incubation 12 h, respectively. Cell viability was determined using the CCK-8 assay and data are expressed as percentage of the control. (C) Representative images of flow cytometric analysis by Annexin V-FITC/PI staining. The bottom right quadrant represents Annexin V-FITC-stained cells (early-phase apoptotic cells) and the top right quadrant represents PI- and FITC-dual-stained cells (late-phase apoptotic or necrotic cells). (D–G) Quantitative determination of early apoptotic cell death as the number of Annexin V-FITC-positive cells with PI-negative cells, and late apoptotic/necrotic cell death as the number of Annexin V-FITC-positive and PI-positive cells. Values are the means ± SD of at least three independent experiments. *P < 0.05 versus control; #P < 0.05 versus 5 μMH2O2 group.
Figure 4. Resveratrol enhanced mitochondrial superoxide generation and the loss of mitochondrial membrane potential in FLSs.
Cells were treated with different concentrations (0, 5, 10, 20, 40 and 80 μM) H2O2 for 12 h and cells were pretreated for 24 h with various concentrations of Res (0, 50, 100, 200 and 400 μM) before treatment with 5 μMH2O2 incubation 12 h, respectively. (A) The mitochondrial superoxide levels were detected using MitoSOX Red and the fluorescence images was carried out using confocal laser scanning microscopy. (B) Δψm was determined using confocal laser scanning microscopy. Red fluorescence represents JC-1 aggregates in normal mitochondria, whereas green fluorescence represents JC-1 monomers, indicating unhealthy mitochondria. Merged images indicate JC-1 aggregates and monomers. (C) The histogram show quantification of the mitochondrial superoxide levels expressed as the percentage change relative to the control group. (D) Δψm in each group was estimated as the red/green fluorescence. Values are the means ± SD of at least three independent experiments. *P < 0.05 versus control; #P < 0.05 versus 5 μMH2O2 group.
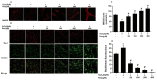
Figure 5. Resveratrol declined autophagy related Beclin1 and LC3A/B proteins expression.
Cells were treated with different concentrations (0, 5, 10, 20, 40 and 80 μM) H2O2 for 12 h and cells were pretreated for 24 h with various concentrations of res (0, 50, 100, 200 and 400 μM) before treatment with 5 μMH2O2 incubation 12 h, respectively. (A,B) Representative images show Beclin1 and LC3A/B proteins localization in FLSs by immunofluorescence assay. Green fluorescence indicates Beclin1 and LC3A/B. Blue fluorescence indicates nucleistained with DAPI. (C) After the indicated treatments, the protein was harvested to detect Beclin1 and LC3A/B levels by western blot analysis. (D,E) Beclin1/GADPH and LC3A/B/GAPDH. Values are the means ± SD of at least three independent experiments. *P < 0.05 versus control;#P < 0.05 versus 5 μMH2O2 group.
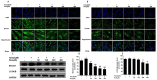
Figure 6. Resveratrol enhanced mtROS generation through declining mitochondrial oxidative stress related proteins SIRT3 and MnSOD expression.
Cells were treated with different concentrations (0, 5, 10, 20, 40, 80 and 100 μM) H2O2 for 12 h and cells were pretreated for 24 h with various concentrations of Res (0, 50,100,200,300 and 400 μM) before treatment with 5 μMH2O2 incubation 12 h, respectively. (A,B) Representative images show mitochondrial SIRT3 and MnSOD proteins localization in FLSs by immunofluorescence assay. Green fluorescence indicates SIRT3 and MnSOD. Blue fluorescence indicates nucleistained with DAPI. (C) After the indicated treatments, the protein was harvested to detect SIRT3 and MnSOD levels by western blot analysis. (D,E) SIRT3/GADPH and MnSOD/GAPDH. Values are the means ± SD of at least three independent experiments. *P < 0.05 versus control; #P < 0.05 versus 5 μMH2O2 group.
Similar articles
- Resveratrol alleviates inflammatory injury and enhances the apoptosis of fibroblast‑like synoviocytes via mitochondrial dysfunction and ER stress in rats with adjuvant arthritis.
Lu J, Zheng Y, Yang J, Zhang J, Cao W, Chen X, Fang S. Lu J, et al. Mol Med Rep. 2019 Jul;20(1):463-472. doi: 10.3892/mmr.2019.10273. Epub 2019 May 22. Mol Med Rep. 2019. PMID: 31180523 Free PMC article. - Effect of lentivirus-mediated overexpression or silencing of MnSOD on apoptosis of resveratrol-treated fibroblast-like synoviocytes in rheumatoid arthritis.
Wang T, Wang G, Zhang Y, Zhang J, Cao W, Chen X. Wang T, et al. Eur J Pharmacol. 2019 Feb 5;844:65-72. doi: 10.1016/j.ejphar.2018.12.001. Epub 2018 Dec 4. Eur J Pharmacol. 2019. PMID: 30529106 - Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes.
Zhang Y, Wang G, Wang T, Cao W, Zhang L, Chen X. Zhang Y, et al. Ann N Y Acad Sci. 2019 Dec;1457(1):166-178. doi: 10.1111/nyas.14196. Epub 2019 Sep 1. Ann N Y Acad Sci. 2019. PMID: 31475364 - Resveratrol derivatives as a pharmacological tool.
Biasutto L, Mattarei A, Azzolini M, La Spina M, Sassi N, Romio M, Paradisi C, Zoratti M. Biasutto L, et al. Ann N Y Acad Sci. 2017 Sep;1403(1):27-37. doi: 10.1111/nyas.13401. Epub 2017 Jul 4. Ann N Y Acad Sci. 2017. PMID: 28675763 Review. - Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights.
de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A. de Sá Coutinho D, et al. Int J Mol Sci. 2018 Jun 20;19(6):1812. doi: 10.3390/ijms19061812. Int J Mol Sci. 2018. PMID: 29925765 Free PMC article. Review.
Cited by
- The gut-joint axis in rheumatoid arthritis.
Zaiss MM, Joyce Wu HJ, Mauro D, Schett G, Ciccia F. Zaiss MM, et al. Nat Rev Rheumatol. 2021 Apr;17(4):224-237. doi: 10.1038/s41584-021-00585-3. Epub 2021 Mar 5. Nat Rev Rheumatol. 2021. PMID: 33674813 Review. - Autophagy: An important target for natural products in the treatment of bone metabolic diseases.
Li Z, Li D, Su H, Xue H, Tan G, Xu Z. Li Z, et al. Front Pharmacol. 2022 Nov 18;13:999017. doi: 10.3389/fphar.2022.999017. eCollection 2022. Front Pharmacol. 2022. PMID: 36467069 Free PMC article. Review. - Recent Overview of Resveratrol's Beneficial Effects and Its Nano-Delivery Systems.
Bohara RA, Tabassum N, Singh MP, Gigli G, Ragusa A, Leporatti S. Bohara RA, et al. Molecules. 2022 Aug 12;27(16):5154. doi: 10.3390/molecules27165154. Molecules. 2022. PMID: 36014390 Free PMC article. Review. - Modulation of Autophagy for Controlling Immunity.
Jang YJ, Kim JH, Byun S. Jang YJ, et al. Cells. 2019 Feb 9;8(2):138. doi: 10.3390/cells8020138. Cells. 2019. PMID: 30744138 Free PMC article. Review. - Efficacy and safety of dietary polyphenols in rheumatoid arthritis: A systematic review and meta-analysis of 47 randomized controlled trials.
Long Z, Xiang W, He Q, Xiao W, Wei H, Li H, Guo H, Chen Y, Yuan M, Yuan X, Zeng L, Yang K, Deng Y, Huang Z. Long Z, et al. Front Immunol. 2023 Mar 22;14:1024120. doi: 10.3389/fimmu.2023.1024120. eCollection 2023. Front Immunol. 2023. PMID: 37033930 Free PMC article.
References
- Ward J. R. & Jones R. S. Studies on adjuvant-induced polyarthritis in rats. I. Adjuvant composition, route of injection, and removal of depot site. Arthritis Rheum. 5, 557–564 (1962). - PubMed
- Shin G. C. et al.. Apigenin-induced apoptosis is mediated by reactive oxygen species and activation of ERK1/2 in rheumatoid fibroblast-like synoviocytes. Chem Biol Interact. 182, 29–36 (2009). - PubMed
- Kamanli A. et al.. Plasmalipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem. Funct. 22, 53–57 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources