Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin - PubMed (original) (raw)
Review
Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin
Keita Nishiyama et al. Microorganisms. 2016.
Abstract
Lactic acid bacteria (LAB) are Gram-positive bacteria that are natural inhabitants of the gastrointestinal (GI) tracts of mammals, including humans. Since Mechnikov first proposed that yogurt could prevent intestinal putrefaction and aging, the beneficial effects of LAB have been widely demonstrated. The region between the duodenum and the terminal of the ileum is the primary region colonized by LAB, particularly the Lactobacillus species, and this region is covered by a mucus layer composed mainly of mucin-type glycoproteins. The mucus layer plays a role in protecting the intestinal epithelial cells against damage, but is also considered to be critical for the adhesion of Lactobacillus in the GI tract. Consequently, the adhesion exhibited by lactobacilli on mucin has attracted attention as one of the critical factors contributing to the persistent beneficial effects of Lactobacillus in a constantly changing intestinal environment. Thus, understanding the interactions between Lactobacillus and mucin is crucial for elucidating the survival strategies of LAB in the GI tract. This review highlights the properties of the interactions between Lactobacillus and mucin, while concomitantly considering the structure of the GI tract from a histochemical perspective.
Keywords: adhesion; carbohydrate; colonization; gastrointestinal tract; histochemistry; lactic acid bacteria; mucin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
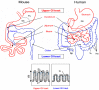
Figure 1
Mouse and human abdominal anatomy. The gastrointestinal (GI) tract is divided into two regions: the upper (red) and the lower (blue) GI tract. The rectangles show schematic models of the villi of the upper and lower GI tracts. G, goblet cells; P, Paneth cells; Fs, forestomach; Gs, glandular stomach; Ap, appendix; Tc, taenia coli; Ha, haustra.
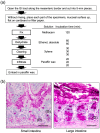
Figure 2
Histology of the mouse small and large intestines. (a) Methacarn fixative and paraffin embedding procedure and methods, based on Puchtler’s protocol [41]. (b) Transverse sections of a 12-week-old male mouse small intestine (jejunum) and large intestine (colon). Methacarn fixative, periodic acid-Schiff stain. OM, outer mucus; IM, inner mucus; G, goblet cells; P, Paneth cells. Scale bars: 100 µm. Based on the results of Nishiyama et al. [47].
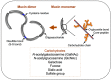
Figure 3
Polymeric structure of mucin molecules. A simplified scheme showing the composition of mucin glycoproteins, in monomer and dimer forms. Glycosylated regions are shown as black diagonal tubes, in which carbohydrate chains form a closely packed sheath around the central peptide core.
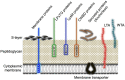
Figure 4
Cell-surface architecture of Gram-positive bacteria. A thick, multilayered peptidoglycan layer is decorated with lipoteichoic acid (LTA), wall teichoic acid (WTA), and various proteins, including S-layer proteins. Cell wall-anchored proteins are attached to the cell wall either (i) covalently by sortases (e.g., LPXTG proteins) or (ii) non-covalently (e.g., through a LysM motif or cell wall-binding domains [CWBDs]). Membrane proteins can covalently attach to the long-chain fatty acids of the cytoplasmic membrane, whereas S-layer proteins are attached to the cell wall through charged or uncharged secondary cell wall polymers. Reproduced from Desvaux et al. [67] with several modifications.
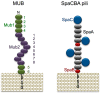
Figure 5
Schematic model of MUB and SpaCBA pili. MUB comprises 6 Mub1 repeats (green) and 8 Mub2 repeats (purple). The C-terminal LPXTG motif anchors MUB to the peptidoglycan of the bacterial cell wall. Reproduced from Roos and Jonsson [68] and Etzold et al. [70] with several modifications. The heterotrimeric SpaCBA pili are composed of shaft-forming SpaA (gray) major pilins together with SpaB (red) and SpaC (navy) minor pilins. Reproduced from Kankainen et al. [76] and Reunanen et al. [77] with several modifications.
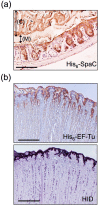
Figure 6
Histochemical staining of the Methacarn-fixed GI mucosal surface with recombinant proteins. (a) Binding of His6-SpaC was observed on the murine colonic mucosa. Arrows indicate the gut contents (C) and the mucus layer (M). Scale bar: 200 µm. Based on the results of Nishiyama et al. [47]. (b) Binding of His6-EF-Tu was observed on the porcine gastric mucosal surface (upper panel). These areas were coincident with areas positively stained for high iron diamine (HID) (lower panel). Scale bars: 1000 µm. Based on the results of Nishiyama et al. [86].
Similar articles
- Studies on the Probiotic, Adhesion, and Induction Properties of Artisanal Lactic Acid Bacteria: to Customize a Gastrointestinal Niche to Trigger Anti-obesity Functions.
Kamber A, Bulut Albayrak C, Harsa HS. Kamber A, et al. Probiotics Antimicrob Proteins. 2024 Oct 9. doi: 10.1007/s12602-024-10357-6. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39382740 - Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells.
Kim Y, Kim SH, Whang KY, Kim YJ, Oh S. Kim Y, et al. J Microbiol Biotechnol. 2008 Jul;18(7):1278-85. J Microbiol Biotechnol. 2008. PMID: 18667857 - Adhesion of Lactobacilli and their anti-infectivity potential.
Yadav AK, Tyagi A, Kumar A, Panwar S, Grover S, Saklani AC, Hemalatha R, Batish VK. Yadav AK, et al. Crit Rev Food Sci Nutr. 2017 Jul 3;57(10):2042-2056. doi: 10.1080/10408398.2014.918533. Crit Rev Food Sci Nutr. 2017. PMID: 25879917 Review. - Dietary Nutrients, Proteomes, and Adhesion of Probiotic Lactobacilli to Mucin and Host Epithelial Cells.
Celebioglu HU, Svensson B. Celebioglu HU, et al. Microorganisms. 2018 Aug 21;6(3):90. doi: 10.3390/microorganisms6030090. Microorganisms. 2018. PMID: 30134518 Free PMC article. Review.
Cited by
- Genomic and Phenotypic Insight into the Probiotic Potential of Lactic Acid Bacterial spp. Associated with the Human Gut Mucosa.
Aziz K, Gilbert JA, Zaidi AH. Aziz K, et al. Probiotics Antimicrob Proteins. 2023 Dec 9. doi: 10.1007/s12602-023-10193-0. Online ahead of print. Probiotics Antimicrob Proteins. 2023. PMID: 38070037 - Quantifying and Engineering Mucus Adhesion of Probiotics.
Mays ZJS, Chappell TC, Nair NU. Mays ZJS, et al. ACS Synth Biol. 2020 Feb 21;9(2):356-367. doi: 10.1021/acssynbio.9b00356. Epub 2020 Jan 13. ACS Synth Biol. 2020. PMID: 31909976 Free PMC article. - The Effects of Cellular Membrane Damage on the Long-Term Storage and Adhesion of Probiotic Bacteria in Caco-2 Cell Line.
Kiepś J, Juzwa W, Olejnik A, Sip A, Tomaszewska-Gras J, Dembczyński R. Kiepś J, et al. Nutrients. 2023 Aug 7;15(15):3484. doi: 10.3390/nu15153484. Nutrients. 2023. PMID: 37571422 Free PMC article. - Histamine release from intestinal mast cells induced by staphylococcal enterotoxin A (SEA) evokes vomiting reflex in common marmoset.
Ono HK, Hirose S, Narita K, Sugiyama M, Asano K, Hu DL, Nakane A. Ono HK, et al. PLoS Pathog. 2019 May 21;15(5):e1007803. doi: 10.1371/journal.ppat.1007803. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31112582 Free PMC article. - Genomic insights into antibiotic-resistance and virulence genes of Enterococcus faecium strains from the gut of Apis mellifera.
Zaghloul HAH, El Halfawy NM. Zaghloul HAH, et al. Microb Genom. 2022 Nov;8(11):mgen000896. doi: 10.1099/mgen.0.000896. Microb Genom. 2022. PMID: 36374179 Free PMC article.
References
- Purchiaroni F., Tortora A., Gabrielli M., Bertucci F., Gigante G., Ianiro G., Ojetti V., Scarpellini E., Gasbarrini A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013;17:323–333. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous