Drug Discovery via Human-Derived Stem Cell Organoids - PubMed (original) (raw)
Review
Drug Discovery via Human-Derived Stem Cell Organoids
Fangkun Liu et al. Front Pharmacol. 2016.
Abstract
Patient-derived cell lines and animal models have proven invaluable for the understanding of human intestinal diseases and for drug development although both inherently comprise disadvantages and caveats. Many genetically determined intestinal diseases occur in specific tissue microenvironments that are not adequately modeled by monolayer cell culture. Likewise, animal models incompletely recapitulate the complex pathologies of intestinal diseases of humans and fall short in predicting the effects of candidate drugs. Patient-derived stem cell organoids are new and effective models for the development of novel targeted therapies. With the use of intestinal organoids from patients with inherited diseases, the potency and toxicity of drug candidates can be evaluated better. Moreover, owing to the novel clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9 genome-editing technologies, researchers can use organoids to precisely modulate human genetic status and identify pathogenesis-related genes of intestinal diseases. Therefore, here we discuss how patient-derived organoids should be grown and how advanced genome-editing tools may be applied to research on modeling of cancer and infectious diseases. We also highlight practical applications of organoids ranging from basic studies to drug screening and precision medicine.
Keywords: inflammatory bowel disease; intestinal cancer; organoid; pluripotent stem cells.
Figures
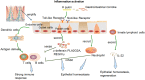
FIGURE 1
The epithelial innate immune response and its effect on epithelial homeostasis, regeneration, inflammation, and gastric carcinogenesis after activation of inflammation by a pathogen. Epithelial cells can express pathogen recognition receptors to pass microbial signals (Nigro et al., 2014; Amieva and Peek, 2016) and generate various peptides against infection and prevent a strong T-cell-mediated immune response (Farin et al., 2014; Leslie et al., 2015); IEC-produced cytokines and dendritic cells can promote T-cell maturation and differentiation (Liu Y. et al., 2015); innate lymphoid cells can produce IL-22 and then regulate ISC survival and proliferation (Bermudez-Brito et al., 2013; Lindemans et al., 2015).
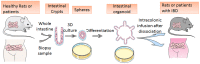
FIGURE 2
Schematic diagram of mini-gut culture and application to stem cell transplantation. The image shows organoid development from isolated crypts to round spheres and budding organoids. After the cultured and dissociated organoids are transplanted into animal models or human bodies, they spontaneously move toward the damaged focus of the DSS-colitis ulcer and promote reconstruction of the epithelial structure.
Similar articles
- Applications of kidney organoids derived from human pluripotent stem cells.
Kim YK, Nam SA, Yang CW. Kim YK, et al. Korean J Intern Med. 2018 Jul;33(4):649-659. doi: 10.3904/kjim.2018.198. Epub 2018 Jun 28. Korean J Intern Med. 2018. PMID: 29961307 Free PMC article. Review. - Human stem cell-based disease modeling: prospects and challenges.
Johnson JZ, Hockemeyer D. Johnson JZ, et al. Curr Opin Cell Biol. 2015 Dec;37:84-90. doi: 10.1016/j.ceb.2015.10.007. Epub 2015 Nov 11. Curr Opin Cell Biol. 2015. PMID: 26546888 Review. - CRISPR/Cas 9 genome editing and its applications in organoids.
Driehuis E, Clevers H. Driehuis E, et al. Am J Physiol Gastrointest Liver Physiol. 2017 Mar 1;312(3):G257-G265. doi: 10.1152/ajpgi.00410.2016. Epub 2017 Jan 26. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28126704 Review. - Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells.
Ho BX, Pek NMQ, Soh BS. Ho BX, et al. Int J Mol Sci. 2018 Mar 21;19(4):936. doi: 10.3390/ijms19040936. Int J Mol Sci. 2018. PMID: 29561796 Free PMC article. Review. - Human Organoid and Supporting Technologies for Cancer and Toxicological Research.
Sekine K. Sekine K. Front Genet. 2021 Oct 22;12:759366. doi: 10.3389/fgene.2021.759366. eCollection 2021. Front Genet. 2021. PMID: 34745227 Free PMC article. Review.
Cited by
- Stem cell modeling of nervous system tumors.
Furnari FB, Anastasaki C, Bian S, Fine HA, Koga T, Le LQ, Rodriguez FJ, Gutmann DH. Furnari FB, et al. Dis Model Mech. 2024 Feb 1;17(2):dmm050533. doi: 10.1242/dmm.050533. Epub 2024 Feb 14. Dis Model Mech. 2024. PMID: 38353122 Free PMC article. Review. - In vitro and in silico Models to Study Mosquito-Borne Flavivirus Neuropathogenesis, Prevention, and Treatment.
Chesnut M, Muñoz LS, Harris G, Freeman D, Gama L, Pardo CA, Pamies D. Chesnut M, et al. Front Cell Infect Microbiol. 2019 Jul 9;9:223. doi: 10.3389/fcimb.2019.00223. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31338335 Free PMC article. Review. - Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest.
Costa J, Ahluwalia A. Costa J, et al. Front Bioeng Biotechnol. 2019 Jun 18;7:144. doi: 10.3389/fbioe.2019.00144. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31275931 Free PMC article. Review. - Gene Signature-Based Approach Identified MEK1/2 as a Potential Target Associated With Relapse After Anti-TNFα Treatment for Crohn's Disease.
Gamo K, Okuzono Y, Yabuki M, Ochi T, Sugimura K, Sato Y, Sagara M, Hayashi H, Ishimura Y, Nishimoto Y, Murakawa Y, Shiokawa Z, Gotoh M, Miyazaki T, Ebisuno Y. Gamo K, et al. Inflamm Bowel Dis. 2018 May 18;24(6):1251-1265. doi: 10.1093/ibd/izy079. Inflamm Bowel Dis. 2018. PMID: 29669006 Free PMC article. - Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models.
Antill-O'Brien N, Bourke J, O'Connell CD. Antill-O'Brien N, et al. Materials (Basel). 2019 Oct 1;12(19):3218. doi: 10.3390/ma12193218. Materials (Basel). 2019. PMID: 31581436 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources