Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems - PubMed (original) (raw)
Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems
Anisur Rahman et al. Physiol Mol Biol Plants. 2016 Jul.
Abstract
Hydroponically grown 12-day-old rice (Oryza sativa L. cv. BRRI dhan47) seedlings were exposed to 150 mM NaCl alone and combined with 0.5 mM MnSO4. Salt stress resulted in disruption of ion homeostasis by Na+ influx and K+ efflux. Higher accumulation of Na+ and water imbalance under salinity caused osmotic stress, chlorosis, and growth inhibition. Salt-induced ionic toxicity and osmotic stress consequently resulted in oxidative stress by disrupting the antioxidant defense and glyoxalase systems through overproduction of reactive oxygen species (ROS) and methylglyoxal (MG), respectively. The salt-induced damage increased with the increasing duration of stress. However, exogenous application of manganese (Mn) helped the plants to partially recover from the inhibited growth and chlorosis by improving ionic and osmotic homeostasis through decreasing Na+ influx and increasing water status, respectively. Exogenous application of Mn increased ROS detoxification by increasing the content of the phenolic compounds, flavonoids, and ascorbate (AsA), and increasing the activities of monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), superoxide dismutase (SOD), and catalase (CAT) in the salt-treated seedlings. Supplemental Mn also reinforced MG detoxification by increasing the activities of glyoxalase I (Gly I) and glyoxalase II (Gly II) in the salt-affected seedlings. Thus, exogenous application of Mn conferred salt-stress tolerance through the coordinated action of ion homeostasis and the antioxidant defense and glyoxalase systems in the salt-affected seedlings.
Keywords: Methylglyoxal; Nutrient homeostasis; Osmotic stress; Oxidative stress; Reactive oxygen species; Trace elements.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
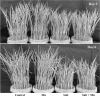
Fig. 1
Phenotypic appearance of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively
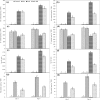
Fig. 2
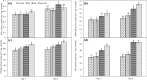
Effect on Na+ and K+ content and their ratio and Mn content in root (a, c, e, g) and leaf (b, d, f, h) of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
Fig. 3
Histochemical detection of O2·- (a) and H2O2 (b) in leaf of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively
Fig. 4
Histochemical detection of lipid peroxidation (a) and loss of plasma membrane integrity (b) in root of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively
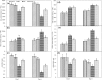
Fig. 5
Effect on AsA content (a), DHA content (b), AsA/DHA ratio (c), GSH content (d), GSSG content (e), GSH/GSSG ratio (f) of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
Fig. 6
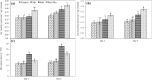
Effect on total phenol content (a) and flavonoid content (b) of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
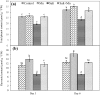
Fig. 7
Effect on APX (a) MDHAR (b), DHAR (c) and GR (d) activities of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
Fig. 8
Effect on SOD (a), CAT (b), GPX (c) and GST (d) activities of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
Fig. 9
Effect on Gly I (a) and Gly II (b) activity, and MG content (c) of rice seedlings under salt stress with and without exogenous Mn. Here, Mn and Salt indicate 0.5 mM MnSO4 and 150 mM NaCl, respectively. Means (±SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the Fisher’s LSD test
Similar articles
- Calcium Supplementation Improves Na(+)/K(+) Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings.
Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Rahman A, et al. Front Plant Sci. 2016 May 12;7:609. doi: 10.3389/fpls.2016.00609. eCollection 2016. Front Plant Sci. 2016. PMID: 27242816 Free PMC article. - Calcium Mitigates Arsenic Toxicity in Rice Seedlings by Reducing Arsenic Uptake and Modulating the Antioxidant Defense and Glyoxalase Systems and Stress Markers.
Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M. Rahman A, et al. Biomed Res Int. 2015;2015:340812. doi: 10.1155/2015/340812. Epub 2015 Dec 20. Biomed Res Int. 2015. PMID: 26798635 Free PMC article. - Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis.
Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Rahman A, et al. C R Biol. 2016 Nov-Dec;339(11-12):462-474. doi: 10.1016/j.crvi.2016.08.002. Epub 2016 Sep 20. C R Biol. 2016. PMID: 27662772 - Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties.
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. Hasanuzzaman M, et al. Biomed Res Int. 2014;2014:757219. doi: 10.1155/2014/757219. Epub 2014 Jun 3. Biomed Res Int. 2014. PMID: 24991566 Free PMC article. - Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants.
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Hasanuzzaman M, et al. Int J Mol Sci. 2017 Jan 20;18(1):200. doi: 10.3390/ijms18010200. Int J Mol Sci. 2017. PMID: 28117669 Free PMC article. Review.
Cited by
- Anatomical and physiological responses of Aechmea blanchetiana (Bromeliaceae) induced by silicon and sodium chloride stress during in vitro culture.
Cipriano R, Martins JPR, Conde LT, da Silva MM, Silva DM, Gontijo ABPL, Falqueto AR. Cipriano R, et al. PeerJ. 2023 Jan 11;11:e14624. doi: 10.7717/peerj.14624. eCollection 2023. PeerJ. 2023. PMID: 36647445 Free PMC article. - A Review of Integrative Omic Approaches for Understanding Rice Salt Response Mechanisms.
Ullah MA, Abdullah-Zawawi MR, Zainal-Abidin RA, Sukiran NL, Uddin MI, Zainal Z. Ullah MA, et al. Plants (Basel). 2022 May 27;11(11):1430. doi: 10.3390/plants11111430. Plants (Basel). 2022. PMID: 35684203 Free PMC article. Review. - Dual Role of Metallic Trace Elements in Stress Biology-From Negative to Beneficial Impact on Plants.
Muszyńska E, Labudda M. Muszyńska E, et al. Int J Mol Sci. 2019 Jun 26;20(13):3117. doi: 10.3390/ijms20133117. Int J Mol Sci. 2019. PMID: 31247908 Free PMC article. Review. - Acetate-induced modulation of ascorbate: glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik.
Hossain MS, Hasanuzzaman M, Sohag MMH, Bhuyan MHMB, Fujita M. Hossain MS, et al. Physiol Mol Biol Plants. 2019 Mar;25(2):443-455. doi: 10.1007/s12298-018-00640-6. Epub 2019 Feb 6. Physiol Mol Biol Plants. 2019. PMID: 30956427 Free PMC article. - Glyoxalase 2: Towards a Broader View of the Second Player of the Glyoxalase System.
Scirè A, Cianfruglia L, Minnelli C, Romaldi B, Laudadio E, Galeazzi R, Antognelli C, Armeni T. Scirè A, et al. Antioxidants (Basel). 2022 Oct 28;11(11):2131. doi: 10.3390/antiox11112131. Antioxidants (Basel). 2022. PMID: 36358501 Free PMC article. Review.
References
- Addinsoft . XLSTAT v. 2015.1.01: data analysis and statistics software for Microsoft Excel. Paris: Addinsoft; 2015.
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. - DOI
- Asada K. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzymes in plants. Physiol Plant. 1992;85:235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x. - DOI
- Ashraf MA, Ashraf M, Ali Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stage: leaf lipid peroxidation and phenolic contents. Pak J Bot. 2010;42(1):559–565.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous