ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions - PubMed (original) (raw)
Miguel A Hernán 2, Barnaby C Reeves 3, Jelena Savović 4, Nancy D Berkman 5, Meera Viswanathan 6, David Henry 7, Douglas G Altman 8, Mohammed T Ansari 9, Isabelle Boutron 10, James R Carpenter 11, An-Wen Chan 12, Rachel Churchill 13, Jonathan J Deeks 14, Asbjørn Hróbjartsson 15, Jamie Kirkham 16, Peter Jüni 17, Yoon K Loke 18, Theresa D Pigott 19, Craig R Ramsay 20, Deborah Regidor 21, Hannah R Rothstein 22, Lakhbir Sandhu 23, Pasqualina L Santaguida 24, Holger J Schünemann 25, Beverly Shea 26, Ian Shrier 27, Peter Tugwell 28, Lucy Turner 29, Jeffrey C Valentine 30, Hugh Waddington 31, Elizabeth Waters 32, George A Wells 33, Penny F Whiting 34, Julian Pt Higgins 35
Affiliations
- PMID: 27733354
- PMCID: PMC5062054
- DOI: 10.1136/bmj.i4919
ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions
Jonathan Ac Sterne et al. BMJ. 2016.
Abstract
Non-randomised studies of the effects of interventions are critical to many areas of healthcare evaluation, but their results may be biased. It is therefore important to understand and appraise their strengths and weaknesses. We developed ROBINS-I (“Risk Of Bias In Non-randomised Studies - of Interventions”), a new tool for evaluating risk of bias in estimates of the comparative effectiveness (harm or benefit) of interventions from studies that did not use randomisation to allocate units (individuals or clusters of individuals) to comparison groups. The tool will be particularly useful to those undertaking systematic reviews that include non-randomised studies.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi\_disclosure.pdf and declare: grants from Cochrane, MRC, and NIHR during the conduct of the study. Dr Carpenter reports personal fees from Pfizer, grants and non-financial support from GSK and grants from Novartis, outside the submitted work. Dr Reeves is a co-convenor of the Cochrane Non-Randomised Studies Methods Group. The authors report no other relationships or activities that could appear to have influenced the submitted work.
Figures
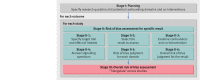
Fig 1 Summary of the process of assessing risk of bias in a systematic review of non-randomised studies of interventions (NRSI)
Similar articles
- The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures.
Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J, Lau A, McDonald S, Mintzes B, Sutton P, Turton JL, Woodruff TJ. Bero L, et al. Syst Rev. 2018 Dec 21;7(1):242. doi: 10.1186/s13643-018-0915-2. Syst Rev. 2018. PMID: 30577874 Free PMC article. - The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions.
Downs SH, Black N. Downs SH, et al. J Epidemiol Community Health. 1998 Jun;52(6):377-84. doi: 10.1136/jech.52.6.377. J Epidemiol Community Health. 1998. PMID: 9764259 Free PMC article. - A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E).
Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, Lemeris C, Akl EA, Bateson TF, Berkman ND, Glenn BS, Hróbjartsson A, LaKind JS, McAleenan A, Meerpohl JJ, Nachman RM, Obbagy JE, O'Connor A, Radke EG, Savović J, Schünemann HJ, Shea B, Tilling K, Verbeek J, Viswanathan M, Sterne JAC. Higgins JPT, et al. Environ Int. 2024 Apr;186:108602. doi: 10.1016/j.envint.2024.108602. Epub 2024 Mar 24. Environ Int. 2024. PMID: 38555664 - The risk of bias of non-randomized observational studies in deep inferior epigastric perforator flap breast reconstruction: A systematic review using ROBINS-I.
Yuan M, Wu J, Lee J, Cao D, Huynh MN, Gallo L, O' Neill AC, Hofer SOP. Yuan M, et al. J Plast Reconstr Aesthet Surg. 2022 Nov;75(11):4096-4105. doi: 10.1016/j.bjps.2022.06.093. Epub 2022 Jun 29. J Plast Reconstr Aesthet Surg. 2022. PMID: 36117133 Review. - Implementation of the trial emulation approach in medical research: a scoping review.
Scola G, Chis Ster A, Bean D, Pareek N, Emsley R, Landau S. Scola G, et al. BMC Med Res Methodol. 2023 Aug 16;23(1):186. doi: 10.1186/s12874-023-02000-9. BMC Med Res Methodol. 2023. PMID: 37587484 Free PMC article. Review.
Cited by
- Molecular Epidemiology of Pseudomonas aeruginosa in Brazil: A Systematic Review and Meta-Analysis.
Rodrigues YC, Silva MJA, Dos Reis HS, Dos Santos PAS, Sardinha DM, Gouveia MIM, Dos Santos CS, Marcon DJ, Aires CAM, Souza CO, Quaresma AJPG, Lima LNGC, Brasiliense DM, Lima KVB. Rodrigues YC, et al. Antibiotics (Basel). 2024 Oct 17;13(10):983. doi: 10.3390/antibiotics13100983. Antibiotics (Basel). 2024. PMID: 39452249 Free PMC article. Review. - Biologic Augmentation Reduces the Failure Rate of Meniscal Repair: A Systematic Review and Meta-analysis.
Zaffagnini S, Poggi A, Reale D, Andriolo L, Flanigan DC, Filardo G. Zaffagnini S, et al. Orthop J Sports Med. 2021 Feb 24;9(2):2325967120981627. doi: 10.1177/2325967120981627. eCollection 2021 Feb. Orthop J Sports Med. 2021. PMID: 33709004 Free PMC article. Review. - Efficacy and safety of artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ethiopia: a systematic review and meta-analysis.
Abamecha A, Yilma D, Adissu W, Yewhalaw D, Abdissa A. Abamecha A, et al. Malar J. 2021 May 6;20(1):213. doi: 10.1186/s12936-021-03745-8. Malar J. 2021. PMID: 33957925 Free PMC article. - The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. Page MJ, et al. Syst Rev. 2021 Mar 29;10(1):89. doi: 10.1186/s13643-021-01626-4. Syst Rev. 2021. PMID: 33781348 Free PMC article. No abstract available. - Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis.
Coleman BL, Sanderson R, Haag MDM, McGovern I. Coleman BL, et al. Influenza Other Respir Viruses. 2021 Nov;15(6):813-823. doi: 10.1111/irv.12871. Epub 2021 Jun 3. Influenza Other Respir Viruses. 2021. PMID: 34081398 Free PMC article.
References
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ 1996;312:1215-8. 10.1136/bmj.312.7040.1215 pmid:8634569. - DOI - PMC - PubMed
- Feinstein AR. An additional basic science for clinical medicine: II. The limitations of randomized trials. Ann Intern Med 1983;99:544-50. 10.7326/0003-4819-99-4-544 pmid:6625387. - DOI - PubMed
- Strom B. Overview of automated databases in pharmacoepidemiology. In: Strom BL, Hennessy S, eds. Pharmacoepidemiology. 5th ed. Wiley, 2012.
- Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666-76. 10.1093/ije/dym018 pmid:17470488. - DOI - PubMed
- Deeks JJ, Dinnes J, D’Amico R, et al. International Stroke Trial Collaborative Group European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii-x, 1-173. 10.3310/hta7270 pmid:14499048. - DOI - PubMed
MeSH terms
Grants and funding
- R35 CA197449/CA/NCI NIH HHS/United States
- HSRU1/CSO_/Chief Scientist Office/United Kingdom
- 16895/CRUK_/Cancer Research UK/United Kingdom
- MR/J004855/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/21/MRC_/Medical Research Council/United Kingdom
- P01 CA134294/CA/NCI NIH HHS/United States
- MC_UU_12023/24/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical