Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse - PubMed (original) (raw)
Yuan Zhang 1, Jun Hua 2, Xiangyu Yang 3, Xiaotian Zhang 1, Ming Duan 4, Xinjian Zhu 1, Wenhui Huang 5, Jie Chao 6, Rongbin Zhou 7, Gang Hu 2, Honghong Yao 1 3
Affiliations
- PMID: 27767041
- PMCID: PMC5073292
- DOI: 10.1038/srep35642
Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse
Ying Bai et al. Sci Rep. 2016.
Abstract
MicroRNA-143 (miR-143) plays a critical role in various cellular processes; however, the role of miR-143 in the maintenance of blood-brain barrier (BBB) integrity remains poorly defined. Silencing miR-143 in a genetic animal model or via an anti-miR-143 lentivirus prevented the BBB damage induced by methamphetamine. miR-143, which targets p53 unregulated modulator of apoptosis (PUMA), increased the permeability of human brain endothelial cells and concomitantly decreased the expression of tight junction proteins (TJPs). Silencing miR-143 increased the expression of TJPs and protected the BBB integrity against the effects of methamphetamine treatment. PUMA overexpression increased the TJP expression through a mechanism that involved the NF-κB and p53 transcription factor pathways. Mechanistically, methamphetamine mediated up-regulation of miR-143 via sigma-1 receptor with sequential activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3' kinase (PI3K)/Akt and STAT3 pathways. These results indicated that silencing miR-143 could provide a novel therapeutic strategy for BBB damage-related vascular dysfunction.
Figures
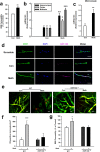
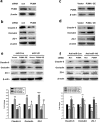
Figure 1. miR-143 is regulated by methamphetamine in the brain and in HBMECs.
(a) BBB permeability was evaluated by measuring the amount of Evans blue extravasation via spectrophotometry of brain tissue at 620 nm. (b,c) Effect of methamphetamine on the mRNA expression of miR-143 in brain tissue from various brain regions, such as the hippocampus, cortex, striatum and midbrain and in microvessels in mice, as determined by real-time PCR. n = 6 animals/group. **p < 0.01 vs. control using Student’s t-test. (d) Fluorescence in situ hybridization of mature miR-143 in microvessels combined with immunostaining for the endothelial cell marker caveolin-1. Red, miR-143; green, caveolin-1; blue, DAPI. Scale bar = 20 μm. (e,f) Representative images from the TPLSM analysis of the methamphetamine-induced transmigration of monocytes from blood vessels in WT and miR-143+/− mice. Scale bar = 50 μm. (g) BBB permeability was evaluated by measuring the amount of brain extravasation of Evans blue in WT and miR-143+/− mice via spectrophotometry at 620 nm. n = 6 animals/group. *p < 0.05 and ***p < 0.001 vs. the WT control group using one-way ANOVA and the Holm-Sidak test. Meth, methamphetamine.
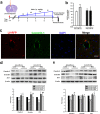
Figure 2. Silencing miR-143 ameliorated the increased permeability of the BBB and endothelial cells in vivo.
(a) Schematic diagram depicting the procedure for the microinjection of the lentivirus into the brain ventricle. Two weeks after the lentivirus injection, the animals in the anti-control and anti-miR-143 lentivirus groups were intraperitoneally injected with either saline or methamphetamine (1.5 mg/kg, 4.5 mg/kg, 7.5 mg/kg, and 10 mg/kg) every day for a total of eight days according to the previously described dosing schedule. (b) The BBB permeability was evaluated by measuring the amount of brain extravasation of Evans blue in the animals microinjected with a lentivirus via spectrophotometry at 620 nm. (c) Representative images of microvessels in C57BL/6 mice microinjected with the RFP lentivirus. Representative images following microinjection of anti-miR-143 into the lateral ventricle. Caveolin-1 staining was conducted 2 weeks later. Green: caveolin-1; Red: RFP. Scale bar = 20 μm. (d,e) Microinjection of anti-miR-143 ameliorated the decreased TJP expression induced by methamphetamine in the cortex (d) and hippocampus (e), as determined by western blot analysis. n = 6 animals/group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the saline+anti-miR-control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. the methamphetamine+anti-miR-control group using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
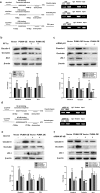
Figure 3. Effect of miR-143 on the permeability of endothelial cells in vitro.
(a) Methamphetamine increased the permeability of the HBMECs. (b) Effect of methamphetamine on the expression of claudin-5, occludin, and ZO-1 in HBMECs evaluated using western blot analysis. (c,d) Effect of miR-143 on the expression of tight junction proteins (c) and the permeability of HBMECs (d). (e,f) Effect of anti-miR-143 on the expression of tight junction proteins (e) and the permeability of HBMECs (f). All data are presented as the mean ± SD of three individual experiments. **p < 0.01 vs. the control group using Student’s t-test. (g) Anti-miR-143 attenuated the methamphetamine-induced increase in the permeability of HBMECs. *p < 0.05 and **p < 0.01 vs. the anti-miR-con/control; ###p < 0.001 vs. the anti-miR-con/meth group using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
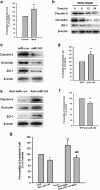
Figure 4. Role of PUMA in the effects of methamphetamine on BBB integrity.
(a) Effect of methamphetamine on the mRNA expression of miR-143 in HBMECs, as determined by real-time PCR. (b) Fluorescence in situ hybridization of mature miR-143 in methamphetamine-treated HBMECs. Red, miR-143; blue, DAPI. Scale bar = 20 μm. (c) Methamphetamine decreased the PUMA expression in HBMECs, as determined by western blot analysis. (d,e) PUMA expression was evaluated at the mRNA (d) and protein (e) level in HBMECs that were transduced with the miR-control/miR-143 and the anti-miR-control/anti-miR-143 lentiviruses. All data are presented as the mean ± SD of three individual experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the control group using one-way ANOVA followed by the Holm-Sidak test. (f,g) Representative images from the TPLSM analysis of the methamphetamine-induced migration of monocytes out of blood vessels in WT and PUMA KO mice. Scale bar = 50 μm. (h) The BBB permeability in WT and PUMA KO mice was determined by measuring the amount of brain extravasation of Evans blue by spectrophotometry at 620 nm. n = 6 animals/group. **p < 0.01 vs. the WT group using Student’s t-test. (i) The BBB permeability in WT and PUMA KO mice microinjected with the anti-miR-control/anti-miR-143 lentivirus was determined by measuring the amount of brain extravasation of Evans blue using spectrophotometry at 620 nm. n = 6 animals/group. **p < 0.01 and ***p < 0.001 vs. the WT mice microinjected with the anti-miR-control lentivirus using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
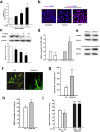
Figure 5. miR-143 regulated the permeability of endothelial cells by targeting PUMA.
(a,b) The transduction of cells with the PUMA siRNA lentivirus decreased the PUMA expression and the expression of tight junction proteins (claudin-5, occludin, and ZO-1) in HBMECs. (c,d) The transduction of HBMECs with the PUMA OE lentivirus increased the expression of PUMA and tight junction proteins (claudin-5, occludin, and ZO-1) in HBMECs. (e) The transduction of cells with miR-143 failed to decrease the level of tight junction proteins in the cells co-transduced with the PUMA OE lentivirus, as determined by western blot analysis. (f) The transduction of cells with the PUMA siRNA lentivirus significantly inhibited the anti-miR-143-induced increase in the expression of tight junction proteins, as determined by western blot analysis. All data are presented as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the miR-Con/Vector group or the anti-miR-Con/siRNA-Con group; +p < 0.05 and ##p < 0.01 vs. the miR-143/Vector group or the anti-miR-143/siRNA-Con group using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
Figure 6. miR-143 induced the activation of the NF-κB and p53 transcription factors by targeting PUMA.
(a) The nuclear translocation of p53 and NF-κB was decreased by the miR-143 lentivirus but increased by the anti-miR-143 lentivirus. (b) The translocation of p53 and NF-κB into the nucleus was decreased by PUMA siRNA decreased but increased by PUMA OE. (c) The PUMA siRNA lentivirus significantly inhibited the anti-miR-143-induced increase in the nuclear translocation of p53 and NF-κB. (d) The PUMA OE lentivirus significantly inhibited the miR-143-induced decrease in the nuclear translocation of p53 and NF-κB. All data are presented as the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 vs. the anti-miR-Con/siRNA-Con group or the miR-Con/Vector group; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. the anti-miR-143/siRNA-Con group or the miR-143/Vector group using one-way ANOVA followed by the Holm-Sidak test.
Figure 7. PUMA regulated the expression of tight junction proteins via pathways involving the transcription factors p53 and NF-κB.
(a) ChIP assay demonstrating the methamphetamine-mediated binding of p53 to the promoters of tight junction proteins (claudin-5, occludin, and ZO-1). (b,c) Pretreatment of HBMECs with the p53 inhibitor PFT-α (10 μM) (b) or p53 siRNA (c) significantly decreased the PUMA OE-induced increase in the level of tight junction proteins. (d) ChIP assay demonstrating the methamphetamine-mediated binding of NF-κB to the promoters of claudin-5 and occludin, but not ZO-1. (e,f) Pretreatment of HBMECs with the NF-κB inhibitor SC-514 (10 μM) (e) or NF-κB siRNA (f) significantly decreased the PUMA OE-induced increase in the expression of claudin-5 and occludin but did not affect the expression of ZO-1. All data are presented as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the Con/vector group; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. the group treated with vector and the inhibitor or siRNA using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
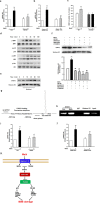
Figure 8. The σ-1R/MAPK/STAT3 pathway was involved in the methamphetamine-induced expression of miR-143.
(a,b) Pretreatment of HBMECs with a σ-1R inhibitor (BD1047, 10 μM) (a) or σ-1R siRNA (b) significantly inhibited the methamphetamine-induced increase in the expression of miR-143, as determined by real-time PCR. (c) Administering methamphetamine to the animals damaged the BBB in WT mice but not in σ-1R KO mice. (d,e) Effect of methamphetamine on the activation of the MAPK and PI3K/Akt cell signaling pathways. (d) Increase in the translocation of STAT3 into the nucleus (e). (f) Pretreatment of HBMECs with an MEK inhibitor (U0126, 10 μM), JNK inhibitor (SP600125, 10 μM), p38 inhibitor (SB203580, 10 μM), and PI3K inhibitor (LY294002, 5 μM) inhibited the methamphetamine-mediated translocation of STAT3 into the nucleus. (g,h) ChIP assay demonstrating methamphetamine-mediated binding of STAT3 to the miR-143 promoter. (I,j) Pretreatment of HBMECs with a STAT3 inhibitor (Stattic, 1 μM) (i) or STAT3 siRNA (j) significantly inhibited the methamphetamine-induced increase in the expression of miR-143. (k) miR-143 regulation of BBB integrity via the targeting of PUMA, the subsequent distinct downstream activation of the p53 and NF-κB pathways, and the cooperative expression of TJPs in endothelial cells. Mechanistically, methamphetamine mediated the up-regulation of miR-143 via the sigma-1 receptor with sequential activation of mitogen-activated protein kinases (MAPKs) and the phosphatidylinositol-3′ kinase (PI3K)/Akt and STAT3 pathways. All data are presented as the mean ± SD of three independent experiments. **p < 0.01 vs. the control, #p < 0.05 and ##p < 0.01 vs. the methamphetamine-treated group using one-way ANOVA followed by the Holm-Sidak test. Meth, methamphetamine.
Similar articles
- Involvement of PUMA in pericyte migration induced by methamphetamine.
Zhang Y, Zhang Y, Bai Y, Chao J, Hu G, Chen X, Yao H. Zhang Y, et al. Exp Cell Res. 2017 Jul 1;356(1):28-39. doi: 10.1016/j.yexcr.2017.04.007. Epub 2017 Apr 10. Exp Cell Res. 2017. PMID: 28408317 - Involvement of NLRP3 inflammasome in methamphetamine-induced microglial activation through miR-143/PUMA axis.
Du L, Shen K, Bai Y, Chao J, Hu G, Zhang Y, Yao H. Du L, et al. Toxicol Lett. 2019 Feb;301:53-63. doi: 10.1016/j.toxlet.2018.10.020. Epub 2018 Oct 28. Toxicol Lett. 2019. PMID: 30394308 - Methamphetamine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells.
Lee YW, Hennig B, Yao J, Toborek M. Lee YW, et al. J Neurosci Res. 2001 Nov 15;66(4):583-91. doi: 10.1002/jnr.1248. J Neurosci Res. 2001. PMID: 11746378 - Regulation of the MIR155 host gene in physiological and pathological processes.
Elton TS, Selemon H, Elton SM, Parinandi NL. Elton TS, et al. Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review. - Possible repair mechanisms of renin-angiotensin system inhibitors, matrix metalloproteinase-9 inhibitors and protein hormones on methamphetamine-induced neurotoxicity.
Zhao W, Zhao YL, Liu M, Liu L, Wang Y. Zhao W, et al. Mol Biol Rep. 2021 Nov;48(11):7509-7516. doi: 10.1007/s11033-021-06741-y. Epub 2021 Oct 8. Mol Biol Rep. 2021. PMID: 34623593 Review.
Cited by
- Dysfunction of the Neurovascular Unit by Psychostimulant Drugs.
Vo TTL, Shin D, Ha E, Seo JH. Vo TTL, et al. Int J Mol Sci. 2023 Oct 13;24(20):15154. doi: 10.3390/ijms242015154. Int J Mol Sci. 2023. PMID: 37894832 Free PMC article. Review. - Targeting microRNAs to Regulate the Integrity of the Blood-Brain Barrier.
Wang J, Xu F, Zhu X, Li X, Li Y, Li J. Wang J, et al. Front Bioeng Biotechnol. 2021 Jun 11;9:673415. doi: 10.3389/fbioe.2021.673415. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34178963 Free PMC article. Review. - Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition.
Yang L, Han B, Zhang Y, Bai Y, Chao J, Hu G, Yao H. Yang L, et al. Autophagy. 2018;14(3):404-418. doi: 10.1080/15548627.2017.1414755. Epub 2018 Jan 29. Autophagy. 2018. PMID: 29260931 Free PMC article. - Temporal changes in mouse hippocampus transcriptome after pilocarpine-induced seizures.
Popova EY, Kawasawa YI, Leung M, Barnstable CJ. Popova EY, et al. Front Neurosci. 2024 Jul 8;18:1384805. doi: 10.3389/fnins.2024.1384805. eCollection 2024. Front Neurosci. 2024. PMID: 39040630 Free PMC article. - MicroRNAs in central nervous system diseases: A prospective role in regulating blood-brain barrier integrity.
Ma F, Zhang X, Yin KJ. Ma F, et al. Exp Neurol. 2020 Jan;323:113094. doi: 10.1016/j.expneurol.2019.113094. Epub 2019 Oct 30. Exp Neurol. 2020. PMID: 31676317 Free PMC article. Review.
References
- Abbott N. J., Ronnback L. & Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). - PubMed
- Abumiya T. et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J. Cereb. Blood Flow Metab. 19, 1038–1050 (1999). - PubMed
- Persidsky Y., Ramirez S. H., Haorah J. & Kanmogne G. D. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 1, 223–236 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous