Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases - PubMed (original) (raw)
Review
Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases
Ulrike Billmeier et al. World J Gastroenterol. 2016.
Abstract
Anti-tumor necrosis factor (TNF) antibodies are successfully used in the therapy of inflammatory bowel diseases (IBD). However, the molecular mechanism of action of these agents is still a matter of debate. Apart from neutralization of TNF, influence on the intestinal barrier function, induction of apoptosis in mucosal immune cells, formation of regulatory macrophages as well as other immune modulating properties have been discussed as central features. Nevertheless, clinically effective anti-TNF antibodies were shown to differ in their mode-of-action in vivo and in vitro. Furthermore, the anti-TNF agent etanercept is effective in the treatment of rheumatoid arthritis but failed to induce clinical response in Crohn's disease patients, suggesting different contributions of TNF in the pathogenesis of these inflammatory diseases. In the following, we will review different aspects regarding the mechanism of action of anti-TNF agents in general and analyze comparatively different effects of each anti-TNF agent such as TNF neutralization, modulation of the immune system, reverse signaling and induction of apoptosis. We discuss the relevance of the membrane-bound form of TNF compared to the soluble form for the immunopathogenesis of IBD. Furthermore, we review reports that could lead to personalized medicine approaches regarding treatment with anti-TNF antibodies in chronic intestinal inflammation, by predicting response to therapy.
Keywords: Apoptosis; Crohn’s disease; Lamina propria mononuclear cells; Mucosal immunology; Transmembrane tumor necrosis factor; Ulcerative colitis.
Conflict of interest statement
Conflict-of-interest statement: Raja Atreya has served as advisor for AbbVie and Markus F Neurath has served as advisor for MSD, AbbVie, Takeda, Boehringer, Giuliani.
Figures
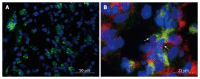
Figure 1
Expression of membrane-bound tumor necrosis factor in the gut. Gut tissue of patients with Crohn’s disease (CD) was cryo-frozen and stained for cell markers by immunofluorescence. Nuclei were counterstained with DAPI. A: Staining for mTNF (green). B: Staining for mTNF (green) and CD14 (red). Co-expressing cells are labeled with an arrow.
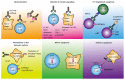
Figure 2
Mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel disease. Schematic illustration of different modes of action of anti-TNF antibodies in inflammatory bowel diseases. TNF: Tumor necrosis factor; mTNF: Membrane-bound TNF; sTNF: Soluble TNF; TNFR: TNF receptor; NK cell: Natural killer cell; ADCC: Antibody-dependent cellular cytotoxicity; CD: Crohn’s disease; CDC: Complement-dependent cytotoxicity; LPS: Lipopolysaccharide.
Similar articles
- Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14⁺ macrophages.
Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD, Rau TT, Rose-John S, Kessler H, Schmidt J, Neurath MF. Atreya R, et al. Gastroenterology. 2011 Dec;141(6):2026-38. doi: 10.1053/j.gastro.2011.08.032. Epub 2011 Aug 27. Gastroenterology. 2011. PMID: 21875498 - Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy.
Caprioli F, Bosè F, Rossi RL, Petti L, Viganò C, Ciafardini C, Raeli L, Basilisco G, Ferrero S, Pagani M, Conte D, Altomare G, Monteleone G, Abrignani S, Reali E. Caprioli F, et al. Inflamm Bowel Dis. 2013 Mar-Apr;19(4):729-39. doi: 10.1097/MIB.0b013e318280292b. Inflamm Bowel Dis. 2013. PMID: 23448791 - Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs.
Zanoli L, Rastelli S, Inserra G, Lentini P, Valvo E, Calcagno E, Boutouyrie P, Laurent S, Castellino P. Zanoli L, et al. Atherosclerosis. 2014 Jun;234(2):346-51. doi: 10.1016/j.atherosclerosis.2014.03.023. Epub 2014 Mar 30. Atherosclerosis. 2014. PMID: 24732573 - Advances in understanding the role of cytokines in inflammatory bowel disease.
Bevivino G, Monteleone G. Bevivino G, et al. Expert Rev Gastroenterol Hepatol. 2018 Sep;12(9):907-915. doi: 10.1080/17474124.2018.1503053. Epub 2018 Jul 30. Expert Rev Gastroenterol Hepatol. 2018. PMID: 30024302 Review. - Safety of anti-tumor necrosis factor therapy during pregnancy in patients with inflammatory bowel disease.
Androulakis I, Zavos C, Christopoulos P, Mastorakos G, Gazouli M. Androulakis I, et al. World J Gastroenterol. 2015 Dec 21;21(47):13205-11. doi: 10.3748/wjg.v21.i47.13205. World J Gastroenterol. 2015. PMID: 26715803 Free PMC article. Review.
Cited by
- Navigating SARS-CoV-2-related immunopathology in Crohn's disease: from molecular mechanisms to therapeutic challenges.
Chen CC, Lin YA, Liu KT, Huang CY, Shih CM, Lee YT, Pan JL, Lee AW. Chen CC, et al. Virol J. 2024 Nov 13;21(1):288. doi: 10.1186/s12985-024-02529-1. Virol J. 2024. PMID: 39538233 Free PMC article. Review. - Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease.
Clua-Ferré L, Suau R, Vañó-Segarra I, Ginés I, Serena C, Manyé J. Clua-Ferré L, et al. Clin Transl Med. 2024 Nov;14(11):e70075. doi: 10.1002/ctm2.70075. Clin Transl Med. 2024. PMID: 39488745 Free PMC article. Review. - Biochemical Modulators of Tight Junctions (TJs): Occludin, Claudin-2 and Zonulin as Biomarkers of Intestinal Barrier Leakage in the Diagnosis and Assessment of Inflammatory Bowel Disease Progression.
Górecka A, Jura-Półtorak A, Koźma EM, Szeremeta A, Olczyk K, Komosińska-Vassev K. Górecka A, et al. Molecules. 2024 Sep 26;29(19):4577. doi: 10.3390/molecules29194577. Molecules. 2024. PMID: 39407507 Free PMC article. - Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems.
Di Rienzo A, Marinelli L, Dimmito MP, Toto EC, Di Stefano A, Cacciatore I. Di Rienzo A, et al. Pharmaceutics. 2024 Sep 7;16(9):1185. doi: 10.3390/pharmaceutics16091185. Pharmaceutics. 2024. PMID: 39339221 Free PMC article. Review. - Sulfate-Reducing Bacteria Induce Pro-Inflammatory TNF-α and iNOS via PI3K/Akt Pathway in a TLR 2-Dependent Manner.
Singh SB, Braun CA, Carroll-Portillo A, Coffman CN, Lin HC. Singh SB, et al. Microorganisms. 2024 Sep 5;12(9):1833. doi: 10.3390/microorganisms12091833. Microorganisms. 2024. PMID: 39338507 Free PMC article.
References
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. - PubMed
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. - PubMed
- Monteleone G, Caprioli F. T-cell-directed therapies in inflammatory bowel diseases. Clin Sci (Lond) 2010;118:707–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical