Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters - PubMed (original) (raw)
Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters
Tokuji Tsuji et al. J Biol Chem. 2017.
Abstract
Zinc-requiring ectoenzymes, including both secreted and membrane-bound enzymes, are considered to capture zinc in their active site for their activation in the early secretory pathway. This idea has been confirmed by our studies conducted using tissue-nonspecific alkaline phosphatase (TNAP), which is elaborately activated by means of a two-step mechanism by zinc transporter 5 (ZNT5)-ZNT6 heterodimers and ZNT7 homodimers, through protein stabilization followed by enzyme activation with zinc in the early secretory pathway. However, the molecular basis of the activation process in other zinc-requiring ectoenzymes remains largely unknown. In this study, we investigated this activation process by using three cancer-promoting zinc-requiring ectoenzymes, autotaxin (ATX), matrix metalloproteinase 9 (MMP9), and carbonic anhydrase IX (CAIX), and the chicken DT40 cell mutants that we generated; we specifically focused on clarifying whether the same or a similar activation mechanism operates in these ectoenzymes. ATX activation required ZNT5-ZNT6 heterodimers and ZNT7 homodimers in a manner similar to TNAP activation, although the protein stability of ATX was differently regulated from that of TNAP. MMP9 required ZNT5-ZNT6 heterodimers and ZNT7 homodimers for its activation as well as secretion; MMP9 was not secreted into the spent medium unless both zinc-transport complexes were present. Finally, CAIX activation by zinc was mediated not only by ZNT5-ZNT6 heterodimers and ZNT7 homodimers but also by ZNT4 homodimers; thus, these three zinc-transport complexes redundantly contribute to CAIX activation. Our results provide pivotal insights into the activation processes of zinc-requiring ectoenzymes, and furthermore, they offer novel insights for potential cancer therapy applications given the cancer-promoting potencies of ATX, MMP9, and CAIX.
Keywords: cancer; enzyme; membrane protein; transporter; zinc.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
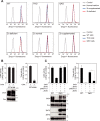
FIGURE 1.
ATX activation, like TNAP activation, requires ZNT5-ZNT6 heterodimers or ZNT7 homodimers. A, exogenously expressed ATX, but not TNAP, converted LPC to LPA (left graph). Conversely, exogenously expressed ATX did not hydrolyze _p_-nitrophenyl phosphate, which is the substrate of TNAP (right graph). B, ATX activity was decreased in DT40 cells lacking Znt5-Znt6 heterodimers (Δ5Δ6) and in TKO cells (left graph), much like endogenous Tnap activity in the same cells (right upper graph). Band intensities of ATX expressed in WT, Δ5Δ6, and TKO cells were quantified by densitometric analysis after normalizing the ATX band intensity to that of Cnx and are shown relative to that of WT cells below each lane. Disruption of each znt gene was confirmed by performing RT-PCR with appropriate primers (right lower panel). C, re-expression of ZNT5-ZNT6 heterodimers or ZNT7 homodimers restored ATX activity in TKO cells (upper graph). D, zinc supplementation did not restore ATX activity in TKO cells. WT or TKO cells stably expressing ATX were cultured in medium supplemented with 0, 25, or 50 μ
m
ZnSO4 for 48 h. Stable expression of ATX was confirmed through immunoblotting (lower panel). E, ZNT5 mutant lacking the PP motif in luminal loop 2 (ZNT5PP-AA) partially restored ATX activity when coexpressed with ZNT6 (left graph) as compared with the activity measured after coexpression of WT ZNT5 with ZNT6; however, expression of the mutant failed to restore Tnap activity (right graph). A–D, ATX and Tnap activities were measured from 10 and 2 μg of membrane proteins, respectively, and representative results from three independent experiments are displayed (*, p < 0.01). Immunoblotting was used to confirm the stable expression of ATX, TNAP, FLAG-ZNT5, ZNT6-HA, and ZNT7-HA. Cnx was used as loading controls. mb. protein, membrane protein.
FIGURE 2.
MMP9 requires ZNT5-ZNT6 heterodimers or ZNT7 homodimer for its activation and secretion into the medium. A, MMP9 activity measured in the spent medium was markedly decreased in the case of TKO cells. The spent medium was prepared by culturing cells for 4 h in medium lacking both FCS and chicken serum. MMP9 activity was examined using gelatin zymography, in which electrophoresis was performed under non-reducing conditions. B, similar to MMP9, MMP2 activity measured in spent medium was also decreased in TKO cells. Gelatin zymography was performed as in A with some minor modifications (see “Experimental Procedures”). A and B, for both WT and TKO cells, the band intensities of MMP9 or MMP2 in the zymography assays and the immunoblots were quantified by densitometric analysis, and the values are shown relative to the WT cells, which were set at 1.0, below each lane. C, secreted ATX protein levels were not significantly different between WT and TKO cells. The serum-free spent media obtained from WT or TKO cells (2 × 106 cells cultured in 500 μl of medium) stably expressing ATX were collected after 4 h of incubation. Total cellular proteins were also prepared from both cells. ATX secreted from the cells (Sec-ATX, upper panel) and present within the cells (Intra-ATX, middle panel) was detected by immunoblotting. D, re-expression of either ZNT5-ZNT6 heterodimers or ZNT7 homodimers in TKO cells restored MMP9 activity and its protein level in the spent medium (top two left panels). In parallel with MMP9 restoration, Tnap activity was restored by re-expression of either ZNT5-ZNT6 heterodimers or ZNT7 homodimers in the same cells (right graph). The band intensities of MMP9 in both the zymography and immunoblots were quantified by densitometric analysis, and the values are shown relative to the WT cells, which were set at 1.0, below each lane. Tnap activity was measured prior to the 4-h incubation of the cells in serum-free medium. E, MMP9 activity (zymograph, upper panel) and protein levels (immunoblot, lower panel) in the serum-free spent media from either WT or TKO cells were increased following zinc supplementation. Cells were cultured for 48 h in normal medium in the presence of 0, 25, or 50 μ
m
ZnSO4 and then cultured for 4 h in serum-free medium containing the same concentrations of ZnSO4. The band intensities of MMP9 in both the zymograph and immunoblot were quantified by densitometric analysis, and the values are shown relative to the WT cells, which were set at 1.0, below each lane. At 50 μ
m
ZnSO4, the MMP9 activity and protein levels in TKO cells were restored to the levels in the WT cells (upper panel). In contrast to MMP9 restoration, Tnap activity was not restored by increasing zinc levels in the same cells (lower graph). F, intracellular MMP9 (intra-MMP9) had a distinct form in TKO cells compared with WT cells. MMP9 was detected at ∼90 kDa in TKO cells and ∼80 kDa in WT cells. Re-expression of the ZNT5-ZNT6 heterodimer or re-expression of the ZNT7 homodimer in TKO cells shifted the MMP9 band from ∼90 to ∼80 kDa. G, treatment with bafilomycin A1 (Baf. A1), but not MG132, changed the MMP9 band size in WT cells from ∼80 to ∼90 kDa. WT and TKO cells were cultured with bafilomycin A1 or MG132 for 4 h. A–G, stable expression of MMP9, MMP2, ATX, FLAG-ZNT5, ZNT6-Myc, and ZNT7-HA was confirmed through immunoblotting. Representative results from three independent experiments are shown. Tubulin and Cnx were used as loading controls. mb. protein, membrane protein.
FIGURE 3.
CAIX can acquire zinc from a pathway independent of ZNT5-ZNT6 heterodimers and ZNT7 homodimers. A, confirmation of endogenous caIX mRNA expression in DT40 cells. Expression of the indicated ca genes was examined by performing RT-PCR with appropriate primers. B, example of the raw data from the ΔpH/Δ_t_ assay used for measuring CAIX activity. Membrane proteins prepared from WT cells and WT cells stably expressing CAIX were used for activity measurement, as described under “Experimental Procedures.” CAIX activity in the membrane proteins isolated from WT cells stably expressing CAIX was completely inhibited upon treatment with the CA inhibitor acetazolamide (ACTZ, 100 μ
m
). The graph also shows endogenous CAIX activity in membrane proteins prepared from parental DT40 cells and ACTZ-treated parental DT40 cells. C, graph showing the calculated CAIX activity (measured as in B). The representative results from three independent experiments are displayed (*, p < 0.01). CAIX protein was detected as broad upper bands at ∼70 kDa and a relatively sharper lower band at ∼60 kDa (lower panel). D, CAIX activity was decreased up to 60% when cells were cultured in zinc-deficient medium (left graph), which is in contrast to the Tnap activity measured in the same cells (right graph). WT cells stably expressing CAIX were cultured in zinc-deficient medium containing Chelex-treated FCS and chicken serum (CX medium) for 24, 48, and 72 h, and CAIX activity and protein were examined. E, decrease in CAIX activity in DT40 cells cultured in zinc-deficient medium was rescued following supplementation with 50 μ
m
ZnSO4 for 48 h (left graph); a similar response was obtained with Tnap activity in the same cells (right graph). D and E show that CAIX protein detected as the broad upper bands at ∼70 kDa were decreased together with the reduction in CAIX activity (lower panels). F, CAIX activity was not markedly altered in TKO cells (left graph), which is in contrast to the large reduction observed in Tnap activity in the same cells (right graph). Consistent with CAIX activity, the broad upper bands at ∼70 kDa were retained (lower panel). C–F, CAIX activity was examined in membrane proteins (200 μg) prepared from the indicated cells. Tnap activity was measured as described in Fig. 1. Representative results from three independent experiments are displayed. Cnx was used as the loading control. mb. protein, membrane protein.
FIGURE 4.
ZNT4 homodimers contribute to CAIX activation redundantly with ZNT5-ZNT6 heterodimers and ZNT7 homodimers. A, znt4 mRNA was detected in DT40 cells. Expression of the indicated znt genes was examined by performing RT-PCR with appropriate primers. B, subcellular localization of ZNT4 in DT40 cells. Cells expressing exogenous ZNT4-FLAG were double-stained with anti-FLAG and anti-GM130. The transmitted light image is also shown. C, ZNT4 forms homodimers. ZNT4-HA and ZNT4-FLAG were immunoprecipitated (IP) with antibodies against FLAG and HA tags, respectively, and the immunoprecipitates were immunoblotted with FLAG and HA tag antibodies. To estimate the amount of tagged ZNT4, 10% of each aliquot was immunoblotted (input panel). D, generation of znt4, znt5, znt6, and znt7 quadruple knock-out cells (znt4_−/−_znt5_−_znt6_−/−_znt7_−/− cells, QKO cells). Disruption of znt4 in TKO cells was confirmed by performing RT-PCR with the appropriate primers. cβ_actin mRNA is shown as an RT-PCR control. E, cell surface protein expression levels were almost identical among WT, TKO, and QKO cells. Biotinylation refers to the biotinylated, solubilized proteins captured using streptavidin beads, and input refers to 10% of the total cell lysate containing biotinylated proteins before avidin capture. The proteins transferred to the PVDF membrane were detected by staining with Coomassie Brilliant Blue (CBB). F, CAIX activity was decreased in QKO cells, but not in _znt4_−/− (Δ4) cells. The zinc-responsive broad upper bands of CAIX were substantially decreased in QKO cells (lower panel). CAIX activity was measured as described in Fig. 3. G–I, reduction in CAIX activity in QKO cells was rescued following the re-expression of ZNT4-HA (G), both FLAG-ZNT5 and ZNT6-Myc (H), or ZNT7-FLAG (I). However, re-expression of the zinc transport-incompetent mutants of the ZNTs (ZNT4H146A-HA, FLAG-ZNT5H451A-ZNT6-Myc, or ZNT7H70A-FLAG, respectively) failed to reverse the activity decrease. Consistent with the restoration of CAIX activity by the aforementioned WT ZNTs, expression of the zinc-responsive broad upper bands of CAIX was also restored. Immunoblotting was used to confirm the stable expression of all ZNT transporters. J, zinc supplementation did not restore either the activity or the zinc-responsive broad upper bands of CAIX in QKO cells. WT and QKO cells stably expressing CAIX were cultured in medium supplemented with 0, 25, or 50 μ
m
ZnSO4 for 48 h. A–J, representative results from three independent experiments are shown (*, p < 0.01 in F–I). Cnx is shown as a loading control. mb. protein, membrane protein.
FIGURE 5.
Biochemical evidence of the functional equivalency of ZNT4 homodimers and ZNT5-ZNT6 heterodimers or ZNT7 homodimers in CAIX activation. A, vesicular zinc levels were not significantly different in QKO cells, compared with WT and TKO cells. Cells were grown in zinc-deficient, normal, or zinc-supplemented (50 μ
m
ZnSO4) medium for 48 h, and then loaded with 5 μ
m
Zinpyr-1. After washing, the cells were analyzed by flow cytometry. Each histogram represents ∼20,000 cells and shows the zinc levels detected by Zinpyr-1 fluorescence. The upper three histograms (left to right) are from WT, TKO, and QKO cells, respectively, and the lower three histograms (left to right) are from the zinc-deficient, normal, and zinc supplemented conditions, respectively. Control, not loaded with Zinpyr-1. B, CAIX activity was not markedly impaired in _znt1_−/−_mt_−/−_znt4_−/− (Δ1M4) cells (left graph), which is in contrast to the large reduction in Tnap activity in the same cells (right graph). The zinc-responsive broad upper bands of CAIX were also not altered in Δ1M4 cells (lower panel). C, CAIX activation, unlike Tnap activation, did not involve the PP motif of ZNT5. Coexpression of ZNT5PP-AA with ZNT6 in QKO cells restored CAIX activity (left graph), but not endogenous Tnap activity (right graph). B and C, CAIX activity was measured as described in Fig. 3, and Tnap activity was measured as described in Fig. 1. Representative results from three independent experiments are displayed. Immunoblotting was used to confirm the stable expression of CAIX and various ZNT transporters. Cnx is shown as a loading control. mb. protein, membrane protein.
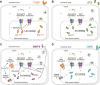
FIGURE 6.
Model for the activation process of TNAP, ATX, MMP9, and CAIX by ZNT transporters in the early secretory pathway. Three ZNT transport complexes, ZNT5-ZNT6 heterodimers, ZNT7 homodimers, and ZNT4 homodimers are localized to the early secretory pathway. The ZNT transport complexes interact with each zinc-requiring ectoenzyme in a unique way. A, TNAP activation process is shown for comparison with ATX, MMP9, and CAIX. ZNT5-ZNT6 heterodimers and ZNT7 homodimers are essential for TNAP activation. TNAP is activated in a two-step mechanism and non-metalated TNAP (apo-TNAP) is degraded intracellularly (8). ZNT4 homodimers do not contribute to its activation (data not shown). B, similar to TNAP, ATX activation requires ZNT5-ZNT6 heterodimers and ZNT7 homodimers. However, in contrast to TNAP, apo-ATX is stable and can be secreted into the extracellular space. ZNT4 homodimers do not contribute to its activation (data not shown). C, MMP9 requires ZNT5-ZNT6 heterodimers and ZNT7 homodimers for its proper maturation and secretion, but this may be complemented by excess zinc supplementation. In this case, other unidentified pathways, including through ZNT4 homodimers, might contribute to the zinc entry route. MMP2 may be regulated in a similar manner. D, CAIX is activated in two manners. The CAIX form that corresponds to the zinc-responsive broad upper bands in immunoblots requires either ZNT5-ZNT6 heterodimers, ZNT7 homodimers, or ZNT4 homodimers for activation, although the CAIX form that corresponds to the sharper lower band in immunoblots does not. Another unknown pathway(s) may be operative as a zinc entry route. A–D, zinc ion is indicated using a yellow dot.
Similar articles
- Detailed analyses of the crucial functions of Zn transporter proteins in alkaline phosphatase activation.
Suzuki E, Ogawa N, Takeda TA, Nishito Y, Tanaka YK, Fujiwara T, Matsunaga M, Ueda S, Kubo N, Tsuji T, Fukunaka A, Yamazaki T, Taylor KM, Ogra Y, Kambe T. Suzuki E, et al. J Biol Chem. 2020 Apr 24;295(17):5669-5684. doi: 10.1074/jbc.RA120.012610. Epub 2020 Mar 16. J Biol Chem. 2020. PMID: 32179649 Free PMC article. - Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells.
Suzuki T, Ishihara K, Migaki H, Ishihara K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Suzuki T, et al. J Biol Chem. 2005 Sep 2;280(35):30956-62. doi: 10.1074/jbc.M506902200. Epub 2005 Jul 1. J Biol Chem. 2005. PMID: 15994300 - The PP-motif in luminal loop 2 of ZnT transporters plays a pivotal role in TNAP activation.
Fujimoto S, Tsuji T, Fujiwara T, Takeda TA, Merriman C, Fukunaka A, Nishito Y, Fu D, Hoch E, Sekler I, Fukue K, Miyamae Y, Masuda S, Nagao M, Kambe T. Fujimoto S, et al. Biochem J. 2016 Sep 1;473(17):2611-21. doi: 10.1042/BCJ20160324. Epub 2016 Jun 14. Biochem J. 2016. PMID: 27303047 Free PMC article. - Activation of zinc-requiring ectoenzymes by ZnT transporters during the secretory process: Biochemical and molecular aspects.
Kambe T, Takeda TA, Nishito Y. Kambe T, et al. Arch Biochem Biophys. 2016 Dec 1;611:37-42. doi: 10.1016/j.abb.2016.03.035. Epub 2016 Apr 1. Arch Biochem Biophys. 2016. PMID: 27046342 Review. - Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway.
Kambe T, Matsunaga M, Takeda TA. Kambe T, et al. Int J Mol Sci. 2017 Oct 19;18(10):2179. doi: 10.3390/ijms18102179. Int J Mol Sci. 2017. PMID: 29048339 Free PMC article. Review.
Cited by
- Zinc deficiency causes delayed ATP clearance and adenosine generation in rats and cell culture models.
Takeda TA, Miyazaki S, Kobayashi M, Nishino K, Goto T, Matsunaga M, Ooi M, Shirakawa H, Tani F, Kawamura T, Komai M, Kambe T. Takeda TA, et al. Commun Biol. 2018 Aug 22;1:113. doi: 10.1038/s42003-018-0118-3. eCollection 2018. Commun Biol. 2018. PMID: 30271993 Free PMC article. - Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review.
Acevedo S, Segovia MF, de la Fuente-Ortega E. Acevedo S, et al. Nutrients. 2024 Jun 26;16(13):2026. doi: 10.3390/nu16132026. Nutrients. 2024. PMID: 38999774 Free PMC article. Review. - Metalation and activation of Zn2+ enzymes via early secretory pathway-resident ZNT proteins.
Kambe T, Wagatsuma T. Kambe T, et al. Biophys Rev (Melville). 2023 Dec 8;4(4):041302. doi: 10.1063/5.0176048. eCollection 2023 Dec. Biophys Rev (Melville). 2023. PMID: 38510844 Free PMC article. Review. - Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology.
Turan B, Tuncay E. Turan B, et al. Int J Mol Sci. 2017 Nov 12;18(11):2395. doi: 10.3390/ijms18112395. Int J Mol Sci. 2017. PMID: 29137144 Free PMC article. Review. - Integrated pan-cancer genomic analysis reveals the role of SLC30A5 in the proliferation, metastasis, and prognosis of hepatocellular carcinoma.
Liu Y, Lu T, Li R, Cui L, Xu R, Teng S, Baranenko D, Zhang T, Yang L, Qie R, Xiao D. Liu Y, et al. J Cancer. 2024 Jul 2;15(14):4686-4699. doi: 10.7150/jca.97214. eCollection 2024. J Cancer. 2024. PMID: 39006068 Free PMC article.
References
- Andreini C., and Bertini I. (2012) A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 111, 150–156 - PubMed
- Maret W. (2012) New perspectives of zinc coordination environments in proteins. J. Inorg. Biochem. 111, 110–116 - PubMed
- Kochańczyk T., Drozd A., and Kreżel A. (2015) Relationship between the architecture of zinc coordination and zinc binding affinity in proteins–insights into zinc regulation. Metallomics 7, 244–257 - PubMed
- Kambe T., Tsuji T., Hashimoto A., and Itsumura N. (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 95, 749–784 - PubMed
- Suzuki T., Ishihara K., Migaki H., Matsuura W., Kohda A., Okumura K., Nagao M., Yamaguchi-Iwai Y., and Kambe T. (2005) Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280, 637–643 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous