Specific Glucoside Transporters Influence Septal Structure and Function in the Filamentous, Heterocyst-Forming Cyanobacterium Anabaena sp. Strain PCC 7120 - PubMed (original) (raw)
. 2017 Mar 14;199(7):e00876-16.
doi: 10.1128/JB.00876-16. Print 2017 Apr 1.
Affiliations
- PMID: 28096449
- PMCID: PMC5350280
- DOI: 10.1128/JB.00876-16
Specific Glucoside Transporters Influence Septal Structure and Function in the Filamentous, Heterocyst-Forming Cyanobacterium Anabaena sp. Strain PCC 7120
Mercedes Nieves-Morión et al. J Bacteriol. 2017.
Abstract
When deprived of combined nitrogen, some filamentous cyanobacteria contain two cell types: vegetative cells that fix CO2 through oxygenic photosynthesis and heterocysts that are specialized in N2 fixation. In the diazotrophic filament, the vegetative cells provide the heterocysts with reduced carbon (mainly in the form of sucrose) and heterocysts provide the vegetative cells with combined nitrogen. Septal junctions traverse peptidoglycan through structures known as nanopores and appear to mediate intercellular molecular transfer that can be traced with fluorescent markers, including the sucrose analog esculin (a coumarin glucoside) that is incorporated into the cells. Uptake of esculin by the model heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 was inhibited by the α-glucosides sucrose and maltose. Analysis of Anabaena mutants identified components of three glucoside transporters that move esculin into the cells: GlsC (Alr4781) and GlsP (All0261) are an ATP-binding subunit and a permease subunit of two different ABC transporters, respectively, and HepP (All1711) is a major facilitator superfamily (MFS) protein that was shown previously to be involved in formation of the heterocyst envelope. Transfer of fluorescent markers (especially calcein) between vegetative cells of Anabaena was impaired by mutation of glucoside transporter genes. GlsP and HepP interact in bacterial two-hybrid assays with the septal junction-related protein SepJ, and GlsC was found to be necessary for the formation of a normal number of septal peptidoglycan nanopores and for normal subcellular localization of SepJ. Therefore, beyond their possible role in nutrient uptake in Anabaena, glucoside transporters influence the structure and function of septal junctions.IMPORTANCE Heterocyst-forming cyanobacteria have the ability to perform oxygenic photosynthesis and to assimilate atmospheric CO2 and N2 These organisms grow as filaments that fix these gases specifically in vegetative cells and heterocysts, respectively. For the filaments to grow, these types of cells exchange nutrients, including sucrose, which serves as a source of reducing power and of carbon skeletons for the heterocysts. Movement of sucrose between cells in the filament takes place through septal junctions and has been traced with a fluorescent sucrose analog, esculin, that can be taken up by the cells. Here, we identified α-glucoside transporters of Anabaena that mediate uptake of esculin and, notably, influence septal structure and the function of septal junctions.
Keywords: ABC transporters; cyanobacteria; glucoside transport; heterocyst; intercellular diffusion; major facilitator superfamily; membrane transporters.
Copyright © 2017 American Society for Microbiology.
Figures
FIG 1
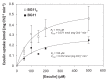
Effect of concentration of esculin on the uptake of esculin by Anabaena. BG11-grown filaments or filaments grown in BG11 medium and incubated for 18 h in BG110 medium were resuspended in the same medium supplemented with 10 mM HEPES-NaOH (pH 7) and used in uptake assays with the indicated concentrations of esculin as described in Materials and Methods. Error bars refer to standard deviations (SD); n = 3.
FIG 2
Effect of pH on the uptake of esculin by Anabaena. Filaments were grown in BG11 medium and then either were resuspended in BG11 medium or were incubated for 18 h in BG110 medium and then resuspended in BG110 medium. Both media were supplemented with 10 mM HEPES-NaOH at the indicated pH values and were used in assays of uptake with 100 μM esculin as described in Materials and Methods. The differences in the mean values of uptake of esculin between filaments resuspended in BG110 and BG11 at the different pH values tested were represented as BG110-BG11. Error bars indicate SD. For pH 7, n = 25 (BG11) or 20 (BG110); for all other pH values, n = 3.
FIG 3
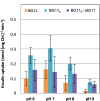
Effect of sugars on the uptake of esculin by Anabaena. BG11-grown filaments or filaments grown in BG11 medium and incubated for 18 h in BG110 medium were resuspended in the same medium supplemented with 10 mM HEPES-NaOH (pH 7) and the indicated sugar at 1 mM. No add., no sugar added; Suc, sucrose; Mal, maltose; Tre, trehalose; Lac, lactose; Glc, glucose; Frc, fructose; Gal, galactose. The assays were performed with 100 μM esculin as described in Materials and Methods. Error bars indicate SD; n = 2 to 3, except for the No add. group (n = 25 [BG11] or 20 [BG110]). Asterisks denote significant differences compared to the assays without added sugars in BG11 or BG110 medium (P < 0.05 by Student's t test).
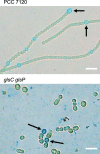
FIG 4
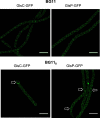
Subcellular localization of GlsC-GFP and GlsP-GFP. Filaments of strains CSMN13 (glsC::gfp) and CSMN15 (glsP::gfp) grown in BG11 medium in the presence of antibiotics were incubated in BG11 or BG110 medium without antibiotics for 24 h. GFP fluorescence was visualized by confocal microscopy as described in Materials and Methods. Brightness and contrast were enhanced to improve visibility. Arrows point to heterocysts. Size bars, 10 μm.
FIG 5
Tests of growth on solid medium of wild-type Anabaena and the glsC (DR3912a), glsP (DR3915), and glsC glsP (DR3912a DR3985a) mutants. Filaments grown in BG11 medium (in the presence of antibiotics for the mutants) were resuspended in BG110 medium, dilutions were prepared, and a 10-μl portion of each dilution (from left to right, 1, 0.5, 0.25, 0.125, and 0.0625 μg Chl ml−1) was spotted on BG11 (NO3−) or BG110 (N2) medium. The plates were incubated under culture conditions, and photographs taken after 7 and 11 days of incubation are shown to help appreciate the growth defect phenotypes.
FIG 6
Heterocysts in the glsC glsP double mutant. Filaments of the wild type (PCC 7120) and of the double mutant grown in BG11 medium (in the presence of antibiotics for the mutant) were inoculated in liquid BG110 medium without antibiotics and incubated for 4 days under culture conditions. Staining with alcian blue was done as described in Materials and Methods, and the filament suspensions were observed by light microscopy. Arrows point to some stained heterocysts. Scale bars, 10 μm.
FIG 7
Subcellular localization of SepJ-GFP in the wild-type and transporter mutant genetic backgrounds. Filaments of strains CSAM137 (PCC 7120 [sepJ::pCSAM137]), CSMN9 (glsC::C.S3 sepJ::pCSVT22), CSMN10 (glsP::C.S3 sepJ::pCSVT22), and CSMN16 (hepP::Tn_5_-1063 sepJ::pCSAM137) were grown in BG11 medium in the presence of antibiotics and visualized by confocal microscopy as described in Materials and Methods. Size bars, 10 µm. In the hepP mutant, SepJ is seen localized in the middle of many cells; these cells are likely starting cell division, and SepJ is known to localize to the cell division site when cell division starts (12, 15).
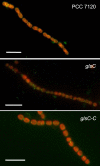
FIG 8
Immunofluorescence localization of SepJ in Anabaena (PCC 7120) and the glsC and glsC-C strains. Filaments of strains PCC 7120, DR3912a (glsC::C.S3), and DR3912a(pCSMN22) (glsC::C.S3 glsC) were grown in BG11 medium in the presence of antibiotics for the mutants and subjected to immunofluorescence analysis with anti-SepJ coiled-coil antibodies as described in Materials and Methods. Overlay images of antibody green fluorescence and cyanobacterial autofluorescence are shown. Size bars, 10 µm.
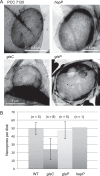
FIG 9
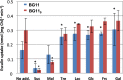
Septal peptidoglycan disk nanopores in wild-type Anabaena and mutants. (A) Murein sacculi were isolated from strains PCC 7120 (WT), DR3912a (glsC::C.S3), DR3915 (glsP::C.S3), and FQ163 (hepP::Tn_5_-1063) grown in BG11 medium and visualized by transmission electron microscopy. (B) Quantification of nanopores in disks from the indicated strains (means and SD; n, number of disks counted). P = 0.002 by Student's t test for WT versus glsC strains.
Similar articles
- Multiple ABC glucoside transporters mediate sugar-stimulated growth in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120.
Nieves-Morión M, Flores E. Nieves-Morión M, et al. Environ Microbiol Rep. 2018 Feb;10(1):40-48. doi: 10.1111/1758-2229.12603. Epub 2017 Dec 14. Environ Microbiol Rep. 2018. PMID: 29159995 - Intercellular diffusion of a fluorescent sucrose analog via the septal junctions in a filamentous cyanobacterium.
Nürnberg DJ, Mariscal V, Bornikoel J, Nieves-Morión M, Krauß N, Herrero A, Maldener I, Flores E, Mullineaux CW. Nürnberg DJ, et al. mBio. 2015 Mar 17;6(2):e02109. doi: 10.1128/mBio.02109-14. mBio. 2015. PMID: 25784700 Free PMC article. - Functional Dependence between Septal Protein SepJ from Anabaena sp. Strain PCC 7120 and an Amino Acid ABC-Type Uptake Transporter.
Escudero L, Mariscal V, Flores E. Escudero L, et al. J Bacteriol. 2015 Aug;197(16):2721-30. doi: 10.1128/JB.00289-15. Epub 2015 Jun 15. J Bacteriol. 2015. PMID: 26078444 Free PMC article. - Roles of DevBCA-like ABC transporters in the physiology of Anabaena sp. PCC 7120.
Shvarev D, Maldener I. Shvarev D, et al. Int J Med Microbiol. 2019 Jul;309(5):325-330. doi: 10.1016/j.ijmm.2019.04.005. Epub 2019 Apr 28. Int J Med Microbiol. 2019. PMID: 31133373 Review. - ATP-binding cassette transporters of the multicellular cyanobacterium Anabaena sp. PCC 7120: a wide variety for a complex lifestyle.
Shvarev D, Maldener I. Shvarev D, et al. FEMS Microbiol Lett. 2018 Feb 1;365(4). doi: 10.1093/femsle/fny012. FEMS Microbiol Lett. 2018. PMID: 29360977 Review.
Cited by
- Heterocyst Septa Contain Large Nanopores That Are Influenced by the Fra Proteins in the Filamentous Cyanobacterium Anabaena sp. Strain PCC 7120.
Arévalo S, Flores E. Arévalo S, et al. J Bacteriol. 2021 Jun 8;203(13):e0008121. doi: 10.1128/JB.00081-21. Epub 2021 Jun 8. J Bacteriol. 2021. PMID: 33846119 Free PMC article. - Cyanobacterial Septal Junctions: Properties and Regulation.
Flores E, Nieves-Morión M, Mullineaux CW. Flores E, et al. Life (Basel). 2018 Dec 20;9(1):1. doi: 10.3390/life9010001. Life (Basel). 2018. PMID: 30577420 Free PMC article. Review. - Phycobilisome protein ApcG interacts with PSII and regulates energy transfer in Synechocystis.
Espinoza-Corral R, Iwai M, Zavřel T, Lechno-Yossef S, Sutter M, Červený J, Niyogi KK, Kerfeld CA. Espinoza-Corral R, et al. Plant Physiol. 2024 Feb 29;194(3):1383-1396. doi: 10.1093/plphys/kiad615. Plant Physiol. 2024. PMID: 37972281 Free PMC article. - Specific mutations in the permease domain of septal protein SepJ differentially affect functions related to multicellularity in the filamentous cyanobacterium Anabaena.
Ramos-León F, Arévalo S, Mariscal V, Flores E. Ramos-León F, et al. Microb Cell. 2018 Oct 16;5(12):555-565. doi: 10.15698/mic2018.12.661. Microb Cell. 2018. PMID: 30533420 Free PMC article. - Coexistence of Communicating and Noncommunicating Cells in the Filamentous Cyanobacterium Anabaena.
Arévalo S, Nenninger A, Nieves-Morión M, Herrero A, Mullineaux CW, Flores E. Arévalo S, et al. mSphere. 2021 Jan 13;6(1):e01091-20. doi: 10.1128/mSphere.01091-20. mSphere. 2021. PMID: 33441411 Free PMC article.
References
- Flores E, Herrero A. 2014. The cyanobacteria: morphological diversity in a photoautotrophic lifestyle. Perspect Phycol 1:63–72. doi:10.1127/pip/2014/0008. - DOI
- Wolk CP, Ernst A, Elhai J. 1994. Heterocyst metabolism and development, p 769–823. In Bryant DA. (ed), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, the Netherlands.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases