C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress - PubMed (original) (raw)
. 2017 Feb 16;542(7641):367-371.
doi: 10.1038/nature21362. Epub 2017 Feb 8.
Marton L Toth 1, Meghan L Arnold 1, Ryan J Guasp 1, Girish Harinath 1, Ken C Nguyen 2, Daniel Taub 3 4, J Alex Parker 5, Christian Neri 6, Christopher V Gabel 3 4, David H Hall 2, Monica Driscoll 1
Affiliations
- PMID: 28178240
- PMCID: PMC5336134
- DOI: 10.1038/nature21362
C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress
Ilija Melentijevic et al. Nature. 2017.
Abstract
The toxicity of misfolded proteins and mitochondrial dysfunction are pivotal factors that promote age-associated functional neuronal decline and neurodegenerative disease. Accordingly, neurons invest considerable cellular resources in chaperones, protein degradation, autophagy and mitophagy to maintain proteostasis and mitochondrial quality. Complicating the challenges of neuroprotection, misfolded human disease proteins and mitochondria can move into neighbouring cells via unknown mechanisms, which may promote pathological spread. Here we show that adult neurons from Caenorhabditis elegans extrude large (approximately 4 μm) membrane-surrounded vesicles called exophers that can contain protein aggregates and organelles. Inhibition of chaperone expression, autophagy or the proteasome, in addition to compromising mitochondrial quality, enhances the production of exophers. Proteotoxically stressed neurons that generate exophers subsequently function better than similarly stressed neurons that did not produce exophers. The extruded exopher transits through surrounding tissue in which some contents appear degraded, but some non-degradable materials can subsequently be found in more remote cells, suggesting secondary release. Our observations suggest that exopher-genesis is a potential response to rid cells of neurotoxic components when proteostasis and organelle function are challenged. We propose that exophers are components of a conserved mechanism that constitutes a fundamental, but formerly unrecognized, branch of neuronal proteostasis and mitochondrial quality control, which, when dysfunctional or diminished with age, might actively contribute to pathogenesis in human neurodegenerative disease and brain ageing.
Conflict of interest statement
Competing interests statement The authors declare that they have no competing financial interests.
Figures
Extended Data Figure 1. Morphological features of exophers derived from touch neurons
a. An exopher is generated with evident filling and growth. S indicates the soma of an ALM neuron on adult day 2 with mCherry visualized; E indicates the significant extrusion of a balloon-like exopher, which grows with time. We noted that the size of this exopher increased for more than an hour, with fluorescence intensity increasing specifically in the exopher compartment, possibly via continual delivery of materials to the exopher after the initial formation (see Supplemental Video 2 for striking time-lapse movie corresponding to this image). Strain is Is[p_mec-4_mCh1]. b. An ALMR soma with multiple exophers. S, soma; E, exopher; strain is Is[p_mec-4_mCh2], adult day 2; scale bar, 2 μm. c. A rare instance of an ALM neuron with exophers that appear extruded from the dendrite (arrows). Strain is Is[p_mec-4_mCh1], adult day 2. Scale bar, 2 μm. d. Size measurements for somas (squares) and exophers (circles). Data are combined for exophers scored in different backgrounds, n = 35. Values are the basis of the measurements in Extended Data Table 2. e. Example of a dye-filled exopher derived from an amphid neuron. We dye-filled the amphid neurons, which are open to the environment, in N2 animals and identified exophers in the amphid region, some of which appeared attached. Neuronal exophers are thus not induced solely in response to expression of foreign proteins, but rather can be produced from neurons that express only native proteins. Scale bar, 2 μm. Note that a second example of an exopher labeled by dye-filling is included in Extended Data Fig. 6c. f. Early adult longitudinal time-course on DiI-soaked wild type N2. Dye-filled chemosensory amphid neurons also produce exophers with a peak early in life in wild type animals. The production of exophers in this study reflects the extrusion of native neuronal contents, as no fluorescent transgene is introduced. Total n > 150, 3 trials; trial means ± s.e.m.; day of adult life indicated on x-axis. g, h. Dopaminergic PDE and CEP neurons can produce exophers. g. GFP-expressing PDE neuron with two anterior exophers indicated (8/450 had exophers, typical of the low rate observed with GFP reporters in touch neurons); h. CEP neuron with an associated exopher. Transgene is dopaminergic neuron-specific reporter egIs1[p_dat-1_GFP]; adult day 2; scale bar, 2 μm. i. ASER neurons can form exophers. Strain is sesIs25 [P_flp-6_Aβ; P_gcy-5_GFP]; adult day 2; scale bar, 2 μm. j. The onset of touch neuron exophers in an hsf-1 mutant background occurs one day earlier than wild type touch neurons, beginning on adult day 1, but follows the general trend of high incidence early in adult life. Longitudinal study with Is[p_mec-4_GFP]; hsf-1(sy441) (2 green trials, starting n = 25), and Is[p_mec-4_mCh1]; hsf-1(sy441) (red, starting n = 25), day of adult life indicated on x-axis. We observed a similar temporal pattern for distinct transgenic line Is[p_mec-4_mCh2]; [p_mec-3_Q128CFP] (data not shown). A late onset peak might not be apparent due to sickness of hsf-1 mutants later in life. Data are from single longitudinal trials and thus error bars are not included.
Extended Data Figure 2. Electron microscopy images of an extruded exopher
a. A membrane GFP reporter reveals that exophers contain membrane. Strain is ZB4524 bzEx242 [P_mec-4_PH(plcDelta)::GFP]. PVM neuron. S, soma; E, exopher. Scale bar, 2 μm. b. General relationship of exopher to ALMR soma. Schematic view from a lateral aspect (anterior to the left). The ALMR soma remains connected to its primary dendrite as represented here, projecting leftward. Several smaller membrane-bound tubes extend away from the soma, containing small items expelled from the ALMR soma, such as the large vesicle shown along the path between the soma and the exopher. The ALMR nucleus (N) remains intact, but pushed to an eccentric position by other cytoplasmic inclusions. The soma contains intact rough endoplasmic reticulum (RER), mitochondria (M), small vesicles (not shown), and larger protein aggregates (A). The exopher comprises heterogeneous contents. The exterior of the exopher is completely bounded by the hypodermal plasma membrane, so that none of the exopher contents are immediately in contact with the hypodermal cytoplasm. Often, a double membrane is observed in which the exopher is likely to supply the inner bilayer, and the hypodermis is likely to contribute the outer bilayer. Additional, separate membrane-bound items lie peripherally (not shown; but see panel h), whose provenance is uncertain, but may represent breakdown products from an earlier, larger stage of the exopher. Most internal contents of the exopher also have their own membrane boundaries, but some diffuse material (not shown; but see panels c, e) fills the spaces around those membrane-bound objects. Membrane-bound contents include portions of the neuronal cytoplasm holding intact RER, large protein aggregates (A), and complex whorls of membrane (W) that generally seem to enclose empty space. Two lysosomes (L) are shown here, one lying separately, nearby the exopher, and a second one in the process of fusing into the exopher’s outer membrane. Other small lysosomes are seen within the exopher (not shown). A tube is shown extending away (far right) towards the pseudocoelom, which might offer one potential route for elimination of contents that cannot be degraded during hypodermal transit. Cartoon designed by and published with permission of C. Crocker. Panelsc,d,f,h are TEM views of a single exopher, emitted from the cell body in panel e. c. The exopher is fully embedded within the hypodermis. Strain is Is[p_mec-4_mCh1]; Is[p_mec-4_GFP]. TEM of thin transverse section, seen from anterior aspect (thus left/right are reversed). Shown is an exopher (E) fully embedded within the hypodermis (H). Animal cuticle shown on the left (C). The exopher is ~1.5 μm across, similar in size to the excretory canal (EC). The exopher lies closer to the pseudocoelom (P), while the ALMR neuron soma lies closer to the cuticle (see panel e). Jagged white spaces running vertically through the hypodermis are an artifact where the tissue cracked during processing. Scale bar, 2 μm. d. Exophers are complex and heterogeneous structures with multiple membranes. The exopher is characterized by many small round protrusions and involutions. The main exopher complex has a complete plasma membrane surrounding it, isolating its contents from the hypodermal cytoplasm. Involuted portions of the exopher can have multiple membrane layers. In this thin section, the exopher displays structures such as protein aggregates (A), rough endoplasmic reticulum (RER), and a possible small lysosome, in addition to loose material floating inside the exopher membrane within a less electron dense fluid matrix. Scale bar, 1 μm. e. The originating touch neuron soma remains intact. The ALMR neuron still has intact cell and nuclear membranes. Small mitochondria (M) are visible inside the soma, as are protein aggregates (A). Note that the aggregate within the soma is not membrane bound, resembling a mammalian aggresome. The electron density of the neuronal cytoplasm is darker than that of the surrounding hypodermis, and the mitochondria of the hypodermis are far larger than those of the neuron. Scale bar, 2 μm. f. The exopher is surrounded by a continuous membrane and contains electron-lucent materials and electron dense membrane whorls (W). The exterior of the exopher is completely bounded by the hypodermal plasma membrane, so that none of the exopher contents are immediately in contact with the hypodermal cytoplasm. The exopher contains complex whorls of membrane (W) that enclose an electron-lucent lumen. Within the exopher membrane, the exopher lumen also is electron-lucent, with some diffuse free-floating material. Scale bar is 1μm. g. Fluorescent images demonstrate exopher features similar to the TEM ultrastructure. Fluorescent microscopy of exophers have irregular shapes and round protrusions, and an irregular distribution of distinct fluorescent signals that resemble the heterogeneous domains we see by TEM. Shown is an exopher in a day 2 animal expressing a soluble YFP, a CFP PolyQ128 fusion, and an aggregation prone mCherry. The mCherry has a bright spot that excludes the other two signals (arrow). The Q128 CFP is localized in the middle portion of the exopher, and the YFP is most strongly localized to the bottom portion of the exopher. The YFP signal also forms a dim ring around the mCherry spot. View is from a lateral aspect, as in panel a. Scale bar, 2 μm. h. Serial sections of the exopher reveal a complex and heterogeneous structure. Seven views (numbered 1–7) are shown from 50 serial thin sections that traverse the main region of the exopher. As one approaches the edge of the exopher, additional membrane-bound objects can be seen at the fringe of the main body (see panels 6, 7), which likely represent portions that have decayed from the original larger object, and are perhaps more easily phagocytosed by the hypodermis, or shuttled along a tube for release into the pseudocoelom. A small electron dense lysosome (L) lies beside the exopher in panel 5. Scale bar, 1 μm.
Extended Data Figure 3. FRAP and post-axotomy calcium imaging support that exophers that appear connected to the soma can fill with cytoplasmic materials
a. Both connected and unconnected exophers can be identified at high frequency in the same strain with a 40x objective. Strain is Is[p_mec-4_mCh1], adult day 2; n = 77 total, 3 trials; trial means ± s.e.m. b. Example of a connected ALMR exopher recovering after laser-bleaching. Strain is Is[p_mec-4_mCh1]; Is[p_mec-4_GFP], adult day 2. 0 seconds is prior to laser treatment, other times are post-treatment. c. Example of a detached ALMR exopher photo-bleaching and failing to refill. Strain is Is[p_mec-4_mCh1]; Is[p_mec-4_GFP], adult day 2. d. Fluorescence recovery measurements reveal that some connected exophers are able to transport fluorescent material from the cytoplasm to the exopher, while disconnected exophers do not appear to transport fluorescent material. Shown are data for examples in b (red, connected) and c (blue, unconnected) above. e. Time-lapse measurements of fluorescence intensity of the soma (blue trace) and the exopher (red trace) during the laser axotomy experiment in f show that the injury-induced calcium wave in the soma is followed by a pulse of calcium elevation in the exopher (yellow arrow, laser axotomy at t = 0 seconds). We analyzed two individual neurons with exophers connected to the soma and three individual neurons with what appeared to be non-connected exophers. Only the clearly connected exophers gave a calcium response comparable (~100% signal) to that measured at the cell soma as in this panel. Strain is ZB4059 bzIs163 [P_mec-4_GCaMP3.0::SL2::mCherry]. f. The soma calcium wave induced by laser axotomy is followed by a calcium wave to connected exophers. We laser-cut an ALMR neuron that had a connected exopher in a day 2 adult that expressed both mCherry and the calcium sensitive fluorophore, GCaMP3. We made the laser cut 20 μm along the axon (yellow arrow) at time t = 0 while taking simultaneous time-lapse images (1 frame/5 seconds). Selected frames are shown at t = −20 seconds (before laser axotomy), right after laser axotomy at t = 5 seconds (note increased fluorescence in the soma; white arrow), and at t = 15 seconds and 25 seconds post-axotomy (note the later exopher increase in fluorescence; white arrowheads). Signal quantitation is in e above. Supplemental Video 3 shows the calcium wave that travels from soma to exopher.
Extended Data Figure 4. Lysosomes can be found in exophers
We also analyzed GFP-tagged lysosomes in touch neurons (bzIs168 [P_mec-7_LMP-1::GFP]) to establish that lysosomes can be extruded in exophers. We observe two types of lysosomal arrangements in exophers: (1) those that have small lysosome-like concentrated fluorescence, with mCherry dispersed (d), and (2) those that are nearly fully loaded with lysosome-like staining in which mCherry is also present throughout (Extended Data Fig. 6c). The inclusion of lysosomes in exophers suggests that some elimination of expelled material might be accomplished via internal degradation. Alternatively, dysfunctional lysosomes might be expelled via exophers. Future studies will be needed to define the role of the lysosome in exophers. a. Neuron soma featuring typical two large LMP-1::GFP-tagged pericentric lysosome domains, with no smaller ones evident. Strain is ZB4509 Is[p_mec-4_mCh1]; bzIs168 [P_mec-7_LMP-1::GFP], green channel shown. As has previously been observed, LMP-1::GFP signal clearly marks the plasma membrane (M), but less intensely than the lysosomes (L). Scale bar, 2 μm. b. LMP-1::GFP reveals lysosome inclusions are frequent and sometimes prominent in exophers. Strain is ZB4070 bzIs168 [P_mec-7_LMP-1::GFP]; S, soma; E, exopher. We found that 18/25 (~70%) of exophers scored contained lysosomes in day 2 adults. Note that LMP-1::GFP faintly labels membrane and rings the exopher in panels b-d, supporting that the exopher is membrane-bound. Scale bar, 2 μm. c, d. Co-labeling of aggregating mCherry and lysosome compartments suggests two types of lysosomal organization in exophers. Strain is ZB4509 Is[p_mec-4_mCh1]; bzIs168 [P_mec-7_LMP-1::GFP]. c. Some exophers appear to be filled with LMP-1::GFP and coincident mCherry. d. LMP-1::GFP-tagged lysosomes included in exophers can be small and differentially localized from mCherry. In the absence of stress, neurons typically feature two large intensely-fluorescent pericentric lysosome domains with LMP-1::GFP, with few smaller ones evident (see panel a). Neurons that had an exopher tended to also have additional small mobile lysosomes that we did not observe in cells without an exopher(see panel b, c, d). Additionally, neurons that featured “large lysosome” exophers generally appeared to have fewer of the large perinuclear lysosomes in the soma (example in d). Scale bar, 2 μm.
Extended Data Figure 5. Mitochondria GFP markers exhibit a normal mitochondrial appearance in exophers
a. Mitochondria in exophers can form a network. Strain Is[P_mec-4_mitoLS::ROGFP]; Is[P_mec-4_mCh1]). Shown is an exopher (E) budding off from the ALMR soma (S). The exopher contains a disproportionate number of punctate mCherry aggregates; the exopher also includes a GFP signal typical in size for neuronal mitochondria. It is noteworthy that the mitochondria in the exopher exhibit a filamentous structure similar to those in the soma, and the signal does not co-localize with the mCherry signal, but rather may remain within a distinct sub-cellular domain. These two observations are consistent with the mito-GFP label localized to actual mitochondria as opposed to representing mislocalized GFP-labeled protein. Scale bar, 2 μm. b. Exophers can contain punctate mitochondria, networked mitochondria, or no mitochondria. Strain Is[P_mec-4_mitoLS::ROGFP]; Is[P_mec-4_mCh1]). Here we show three exophers. In the left exopher, the mitoROGFP signal is localized to two puncta. The middle exopher contains networked mitochondria. The right exopher contains no visible mitoROGFP signal. Scale bar, 2 μm. c. Zoom out of panel b, to show location of exophers(E) relative to the touch neuron soma (S). Scale bar, 2 μm. d. MitoTimer reporter reveals a difference in mitochondrial-matrix oxidation environment in exophers vs. somas. MitoTimer encodes a dsRed derivative that fluoresces green when reduced (first synthesized), but irreversibly shifts to red fluorescence as it oxidizes. We used a single copy p_mec-4_MitoTimer reporter to measure the relative red/green signal in exopher-soma pairs. Exophers have proportionately more oxidized signal, suggesting “older” mitochondria (with more oxidation of the matrix-localized reporter) are preferentially expelled. n = 7 exophers, scale bar, 2 μm; single exopher means ± s.e.m.; *P < 0.05, paired _t_-test. e. Pharmacological disruption of mitochondria leads to higher rates of mitochondrial inclusion in exophers. Juglone exposure leads to an increase in intracellular reactive oxygen species production, most notably superoxide radicals, and can cause mitochondrial membrane depolarization and opening of permeability transition pores, allowing pro-apoptotic Cytochrome C to escape from the mitochondria. We treated strain Is[P_mec-4_mCh1]; zhsEx17 [P_mec-4_mitoLS::ROGFP] with 230 μM juglone which increases ROS production.. Exophers from ALMR neurons from animals exposed to juglone (blue, n = 30 total exophers) were significantly more likely to include at least one mitochondrion than exophers from animals in the control (white, n = 22 total exophers). Mitochondrial extrusion increases under conditions of juglone-induced oxidative stress. 3 trials, trial means ± s.e.m.; **P < 0.01, unpaired _t_-test.
Extended Data Figure 6. RNAi knockdown of ced-1 and ced-6, but not other engulfment machinery, increases occurrence of multiple exophers detected
a. RNAi knockdown of ced-1 and ced-6 engulfment genes increases the number of ALMR neurons with 2 or more exophers near the touch neurons, supporting conclusions from mutants. RNAi against C. elegans transmembrane receptor ced-1/CD91, ced-6/GULP, GTPase ced-10/RAC1, phosphatidylserine receptor psr-1, and pgrn-1/progranulin-1(RNAi increases apoptotic corpse clearance). Control is empty vector, strain is ZB4071 bzIs169 [P_mec-18sid-1_P_sng-1_YFP]; bzIs101 [P_mec-4_mCherry], at least 3 trials each, n > 30 ALMRs measured per trial; n > 15 cells with exopher per condition graphed; means ± s.e.m., one-way ANOVA with Dunnett’s test, *P < 0.05 and **P < 0.01. Note that ced-1 and ced-6 RNAi do not increase the percentage of ALMRs producing exophers (data not shown), but rather increases the number of animals with multiple exophers detectable at adult day 2. Thus, ced-1 and ced-6 knockdown might affect persistence of expelled materials introduced into the hypodermal compartment. b. Phosphatidylserine (PS) indicator annexin V::GFP ref. labels apoptotic corpses, but does not label exophers in ZB4083 smIS76 [p_hsp-16_ANV::GFP]; Is[p_mec-4_mCh1]. Broadly expressed annexinV::GFP binds to PS on developmental apoptotic corpses in embryos (I), but does not bind to exophers (right example compares mCherry-labeled exopher (II) to annexinV::GFP channel for the same image (III), which should visualize bound annexinV::GFP (as occurs for apoptotic corpses in embryos of the same strain (I) if PS is on the exopher surface). Note that in published studies, PS can be recognized on corpses of necrotic touch neurons, showing that touch neurons can produce surface PS, and can be recognized by annexinV-tagging, when inappropriately induced to die,. 0/18 fluorescent mCherry-labeled exophers were co-labeled with annexinV::GFP. Knocking down ced-1 by RNAi in the annexinV::GFP line did not enable us to better detect PS on exophers (n = 0/25 additional observations). c. DiI introduced via amphid neurons can be detected in anterior coelomocytes. In a DiI-soaked N2 animal, an amphid exopher (E) originating from the ASIR soma (S) can be seen proximal to the terminal bulb of the pharynx (P). The anterior coelomocytes ccAR and ccPR (C) also contain DiI. Coelomocytes have no connection to the external environment, suggesting that the DiI must have been uptaken and jettisoned by the chemosensory amphid neurons and subsequently engulfed, analogously to the mCherry detected in Is[pmec-4 mCh1] coelomocytes. This wild type context suggests coelomocytes can scavenge the contents of exophers that are generated under normal physiological conditions, without the added stress of a potentially aggregating product of a transgene. Scale bar 5 μm. d. In ZB4082 cup-4(ok837); Is[p_mec-4_mCh1] mutants in which coelomocyte uptake is disrupted, we observe increased incidence of dispersed fluorescence, similar to that shown here (S, ALMR soma; D bracket, “starry night phenotype,” present in 29/200 animals, adult day 4). Similar dispersions are rare in cup-4(+) lines. Scale bar, 2 μm. AVM soma is also visible. e. Young adult animals that produced an exopher often later exhibit the starry night phenotype, suggesting mCherry material can move through the body. Is[p_mec-4_mCh1] animals were separated into populations that had an ALMR exopher on either adult day 2 or day 3 (blue arrows), and a population that had no ALMR exopher on either day 2 or 3 (white arrows). Animals were scored again on day 5 for presence of the starry night phenotype. In the exopher-producing group, 42% of animals exhibited a starry night phenotype, while in the non-exopher producing group only 6% of the animals exhibited the starry night phenotype. 3 trials, n = 60 total per group, means ± s.e.m.; *P < 0.05, unpaired _t_-test. Arrow thickness is weighted according to relative incidence. Note that the “no exopher” category should include animals that have actually produced exophers, but were not present at the time of sampling, thus differences are likely to be under-estimated in this panel.
Extended Data Figure 7. Working model for a proposed exopher role in proteostasis
As neurotoxic events such as protein aggregation or mitochondrial dysfunction occur in the cell, multiple homeostatic mechanisms clear them (left panel). At the young adult transition point to adult proteostasis (heat shock response down, UPR down, proteasome activity up–) or when basal levels of damage reach a threshold and overwhelm neuronal proteostasis, aggregates and organelles such as mitochondria and lysosomes are sequestered into a compartment that can be jettisoned from the cell. One possibility is that this compartment might correspond to the aggresome described in mammalian cells. For touch neurons, extruded exopher contents may be degraded by accompanying lysosomes, digested by the surrounding hypodermis, or may reach the pseudocoelom and be taken up by coelomocytes. The process of exopher-genesis appears to be neuroprotective in young adults, but when dysregulated, might induce toxicity in neighboring tissues. We speculate that exopher contents that cannot be degraded or passed on could remain in the neighboring cell, where they could contribute to dysfunction. Possibly, exopher-genesis may be akin to the process by which protein aggregates and mitochondria become localized to neighboring neurons in humans, promoting disease spread.
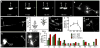
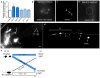
Figure 1. C. elegans touch neurons can extrude cytoplasmic contents
a. An exopher is generated with a striking concentration of fluorescence segregated to the extrusion. Strain is Is[p_mec-4_mCh2]. ALM neuron with mCherry-visualized cytoplasm and aggregates. See supplementary Video 1 for corresponding movie:
. b. Exophers do not include nuclear-like levels of DNA. Blue, DAPI; red, cytoplasm. 0/25 exophers but 25/25 soma were DAPI positive. Strain is Is[p_mec-4_mCh2]. c. An ALMR soma with one attached exopher (right) and one unattached (left). Strain is Is[p_mec-4_mCh1]; Is[p_mec-4_GFP]. d. Individual touch neurons differ in their production of detectable exophers. ALMR neurons produce most (~23%) for Is[p_mec-18sid-1_]; Is[p_mec-4_mCh3], and PLM neurons fail to generate detectable extrusions (0/>500). 12 trials of RNAi empty vector controls with n > 500 total for each neuron. All animals, adult day 2; E, exophers; S, soma; arrowhead, neuronal process; scale bars, 2 μm; trial means ± s.d. Data in d are mean ± s.d.
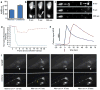
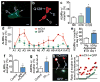
Figure 2. Touch neurons under proteotoxic stress jettison aggregation-prone proteins into exophers
a. CFP-tagged Q128 concentrated into a budding domain. Strain is Is[p_mec-4_mCh2; p_mec-3_Q128CFP]. Blue, Q128CFP; green, mitochondria GFP signal. b. Mature exopher containing Q128CFP aggregates. Strain is Is[p_mec-4_mCh2; p_mec-3_Q128CFP]. 5/10 ALM exophers were Q128CFP positive. These five had no detectable Q128CFP in their somas. c. Neurons expressing Q128CFP produce more exophers than neurons expressing Q19CFP. Is[p_mec-4_GFP] (Q0), Is[p_mec-7_YFP; p_mec-3_Q19CFP] (Q19) and Is[p_mec-7_YFP; p_mec-3_Q128CFP] (Q128). 3 trials, n > 100 total for each strain, one-way ANOVA with Tukey’s test, *P < 0.05. PolyQ-expressing strains have similar expression levels. **d.** Touch neuron exophers are detectable in young adults, diminish in abundance in midlife, and increase again in older animals. Longitudinal study of Is[p_mec-4_GFP] and Is[p_mec-4_mCh1] (starting _n_ = 75 total per strain), 3 trials, adult days 1–12. Variation between days and between strains was significant (***_P_ < 0.001). A similar early adulthood peak occurs in dye-filled amphid neurons (Extended Data Fig. 1f) and in the _hsf-1_ mutant (Extended Data Fig. 1j). **e.** Multiple early visible aggregates predict later exopher formation. On adult day 1, we segregated animals by number of mCherry aggregates (1 Ag vs ≥ 2 Ag) in the ALMR soma and scored for exophers on adult day 2. 5 trials, _n_ > 130 total per condition, ***P < 0.001. Strain is Is[p_mec-4_mCh1]. **f.** Reducing mCherry expression levels using an anti-mCherry RNAi reduces exopher levels. Strain is Is[p_mec-18sid-1_ p_mec-4_mCh3]. 3 trials, _n_ > 35 per trial, *P < 0.05. **g.** ASER neurons expressing human toxic AD protein fragment exhibit elevated exopher production. Adult day 2 exopher production for 7 trials, _n_ > 800 total for each strain, sesIs2512 [P_gcy-5_GFP], and sesIs25 [P_flp-6_Aβ1–42; P_gcy-5_GFP]. *P < 0.05. h, i. Aggregation-prone mCherry is preferentially segregated into the exopher compared to non-aggregating GFP, which is relatively more concentrated in the soma. h. mCherry (top) and GFP (bottom) channels from an ALMR exopher and soma pair in a strain co-expressing Is[p_mec-4_mCh1] and Is[p_mec-_GFP]. i. Quantification of mCherry and GFP fluorescence ratios for exopher and soma pairs. Each point represents an exopher-to-soma fluorescence ratio for either GFP or mCherry. Each cell has a paired GFP and mCherry ratio, aligned vertically, n = 23 pairs. Mean E/S ratio of mCherry was 2.2, mean E/S ratio of GFP was 0.75, **P < 0.01. All animals, adult day 2; E, exophers; S, soma; scale bars, 2 μm; trial means ± s.e.m.; unpaired _t_-test (e, f, g), one-way ANOVA (d) and one-way ANOVA with Tukey’s test (c).
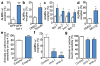
Figure 3. Disruption of multiple branches of proteostasis increases exopher formation
a. Disrupting proteostasis by hsf-1 impairment increases exopher formation. Strains were Is[p_mec-4_GFP] and Is[p_mec-4_GFP]; hsf-1(sy441), ***P < 0.001, _n_ > 280 total per strain. b. Pharmacological inhibition of autophagy by Spautin-1 increases the occurrence of exophers. Strain is Is[p_mec-4_GFP], n > 80 total per condition, *P< 0.05. **c.** RNAi knockdown of autophagy genes _lgg-1_, _atg-7_, and _bec-1_ increases the occurrence of exophers. Strain is Is[p_mec-18sid-1_ p_mec-4_mCh3]. White, empty vector control RNAi; blue, RNAi against gene indicated, _lgg-1_ (5 trials), _atg-7 (_5 trials), _bec-1_ (4 trials)_, lgg-1/2_ (5 trials), _n_ > 100 total per condition, *P< 0.05. **d.** Pharmacological inhibition of the proteasome by MG132 treatment increases the occurrence of exophers. Strain is Is[p_mec-4_mCh1], 3 trials, _n_ > 33 per trial, *P < 0.05. **e.** Q128-expressing animals with an ALMR exopher on day 2 have better anterior touch sensitivity on adult day 4 compared to animals with no apparent early exopher. We tested sensitized strain Is[p_mec-4_mCh2; p_mec-3_Q128CFP], which exhibits accelerated functional decline of touch neurons. 3 trials, _n_ > 100 animals total, ***P < 0.001. Note that the “no exopher” category should include animals that have actually produced exophers, but were not present at the time of sampling, thus differences are likely to be under-estimated in this panel. **f.** Knocking down _pod-1_ or _emb-8_ by RNAi significantly decreases exopher detection, defining a genetic approach to limiting exopher-genesis. Strain was Is[p_mec-18sid-1_ p_mec-4_mCh3]. 4 trials, _n_ > 100 total per condition, ***P< 0.001. **g.** Knocking down _pod-1_ and _emb-8_ by RNAi is associated with a decrease of anterior touch sensitivity in day 4 adults. Strain was Is[p_mec-18sid-1_ p_mec-4_mCh3], an aggregation-prone mCherry line which, like wild type, maintains strong touch sensitivity in young adult life. _pod-1_ and _emb-8_ RNAi knockdown had no effect on young animal touch sensitivity from egg-lay RNAi knockdown, but L4 _pod-1_ and _emb-8_ knockdown reduced exopher production and decreased touch sensitivity in day 4 adults. 3 trials, _n_ > 130 total per condition, *P < 0.05. All animals, adult day 2; E, exophers; S, soma; scale bars, 2 μm; trial means ± s.e.m. (a, b, c,), ± s.d. (d, e, f,); unpaired _t_-test (a, b, c, d, e), one-way ANOVA with Dunnett’s test (f, g).
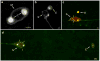
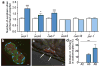
Figure 4. Mitochondria can be extruded in exophers, and mitochondria with higher mitochondrial matrix oxidation might be preferentially extruded
a. Mitochondria in a budding exopher. Mitochondria form a ring around the somatic periphery, typical of young adulthood, with some mitochondria segregated into a putative exopher. Strain is bzIs167[p_mec-4_mitoGFP]. b. Mitochondrially localized mitoGFP (strain bzIs167[P_mec-4_mitoGFP]) can be extruded in exophers; left exopher shown does not include substantial mitochondrial content, but right exopher does. 10/20 exophers scored with this reporter contained mitochondria. c. RNAi knockdown of mitochondrial health and function genes ubl-5, pink-1, and dct-1 increases the occurrence of exophers. White, empty vector control; blue, RNAi against genes indicated. _ubl-5 (_3 trials), _pink-1(_4 trials,) _dct-1 (_3 trials). n > 80 total per condition, *P < 0.05. d. Genetic disruption of mitochondrial health and function increases the occurrence of exophers. We compared exopher levels in Is[p_mec-4_mCh1]; pdr-1(gk448) mutant, a Parkin homolog *P < 0.05, n = 30 per trial, 6 trials. e. Mitochondria segregated into exophers have higher relative oxidation levels than somatic mitochondria, as reported by mitoROGFP. Left, a pseudo-colored image indicating relative emission levels at excitation wavelengths of 405 nm/476 nm (blue, oxidized; green, reduced). Right, redox excitation ratio in exophers vs. soma. n =10 pairs of exophers with mitochondria and originating soma, *P < 0.05, strain is zhsEx17 [P_mec-4_mitoLS::ROGFP]. Of note, the soma shown exhibits locally concentrated oxidized mitochondria, indicating that oxidizing conditions are not restricted to exopher. Wild type, unstressed somas have a typical 405 nm/476 nm ratio of 1; cells that form an exopher may experience somewhat elevated levels of oxidation in the soma, overall. All animals, adult day 2; E, exophers; S, soma; scale bars, 2 μm; trial means ± s.e.m. (c, d, e,), unpaired _t_-test (c, d), one-way ANOVA with Tukey’s test (e).
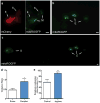
Figure 5. Fluorescent mCherry escapes touch neurons and surrounding hypodermis to later concentrate in distant coelomocytes
a. ced-1, ced-6, and ced-7 mutations increase the number of ALMR neurons with two or more exophers, and ced-5, ced-10, and psr-1 mutations, acting in a parallel phagocytosis pathway, do not. Strain zdIs5[P_mec-4_GFP]), adult day 2, 3 trials, n > 90 total per strain; unpaired _t_-test, *P < 0.05; ***P < 0.001 (replicating RNAi data in Extended Data Fig. 6a). ced-1, ced-6, and ced-7 mutations do not increase the percentage of ALMRs producing exophers (data not shown), but rather increase multiple exopher detection, suggesting a deficit in persistence rather than generation. b. In older animals, coelomocytes (green) can concentrate fluorescent proteins that were originally expressed in touch neurons (red). Strain Is[p_mec-4_mCh1]; pwIs979[Pcup-4GFP::_vps-29_], adult day 6. c. mCherry localization in coelomocytes (arrows) can also be visualized with DIC underlay. Strain is Is[p_mec-4_mCh1], adult day 6. d. The number of coelomocytes containing mCherry released from touch neurons increases with time. Is[p_mec-4_mCh1] animals with an exopher on adult day 2 were segregated and scored for red fluorescence in coelomocytes on A2, A3 and A5; _n_=20 per trial, 3 trials, **P< 0.01, one-way ANOVA with Tukey’s test. Scale bars, 2μm. Data are mean±s.e.m.
Comment in
- Papers of note in Nature542 (7641).
VanHook AM. VanHook AM. Sci Signal. 2017 Feb 21;10(467):eaam9984. doi: 10.1126/scisignal.aam9984. Sci Signal. 2017. PMID: 28223419 - Cell biology of the neuron: Lightening the load.
Bray N. Bray N. Nat Rev Neurosci. 2017 Apr;18(4):195. doi: 10.1038/nrn.2017.27. Epub 2017 Feb 23. Nat Rev Neurosci. 2017. PMID: 28228637 No abstract available. - Exophers expel toxic aggregates: The new discovery of a defense mechanism against misfolded or toxic proteins.
Marvian AT, Morshedi D. Marvian AT, et al. Mov Disord. 2017 Jun;32(6):841. doi: 10.1002/mds.27007. Epub 2017 Apr 10. Mov Disord. 2017. PMID: 28394086 No abstract available.
Similar articles
- Mechanical force of uterine occupation enables large vesicle extrusion from proteostressed maternal neurons.
Wang G, Guasp RJ, Salam S, Chuang E, Morera A, Smart AJ, Jimenez D, Shekhar S, Friedman E, Melentijevic I, Nguyen KC, Hall DH, Grant BD, Driscoll M. Wang G, et al. Elife. 2024 Sep 10;13:RP95443. doi: 10.7554/eLife.95443. Elife. 2024. PMID: 39255003 Free PMC article. - Quantitative Approaches for Scoring in vivo Neuronal Aggregate and Organelle Extrusion in Large Exopher Vesicles in C. elegans.
Arnold ML, Cooper J, Grant BD, Driscoll M. Arnold ML, et al. J Vis Exp. 2020 Sep 18;(163):10.3791/61368. doi: 10.3791/61368. J Vis Exp. 2020. PMID: 33016946 Free PMC article. - Large vesicle extrusions from C. elegans neurons are consumed and stimulated by glial-like phagocytosis activity of the neighboring cell.
Wang Y, Arnold ML, Smart AJ, Wang G, Androwski RJ, Morera A, Nguyen KCQ, Schweinsberg PJ, Bai G, Cooper J, Hall DH, Driscoll M, Grant BD. Wang Y, et al. Elife. 2023 Mar 2;12:e82227. doi: 10.7554/eLife.82227. Elife. 2023. PMID: 36861960 Free PMC article. - Mechanisms of aging-related proteinopathies in Caenorhabditis elegans.
Kim DK, Kim TH, Lee SJ. Kim DK, et al. Exp Mol Med. 2016 Oct 7;48(10):e263. doi: 10.1038/emm.2016.109. Exp Mol Med. 2016. PMID: 27713398 Free PMC article. Review. - Mitochondrial quality control: The role of mitophagy in aging.
Shi R, Guberman M, Kirshenbaum LA. Shi R, et al. Trends Cardiovasc Med. 2018 May;28(4):246-260. doi: 10.1016/j.tcm.2017.11.008. Epub 2017 Dec 6. Trends Cardiovasc Med. 2018. PMID: 29287956 Review.
Cited by
- In situ visualization of endothelial cell-derived extracellular vesicle formation in steady state and malignant conditions.
Atkin-Smith GK, Santavanond JP, Light A, Rimes JS, Samson AL, Er J, Liu J, Johnson DN, Le Page M, Rajasekhar P, Yip RKH, Geoghegan ND, Rogers KL, Chang C, Bryant VL, Margetts M, Keightley MC, Kilpatrick TJ, Binder MD, Tran S, Lee EF, Fairlie WD, Ozkocak DC, Wei AH, Hawkins ED, Poon IKH. Atkin-Smith GK, et al. Nat Commun. 2024 Oct 22;15(1):8802. doi: 10.1038/s41467-024-52867-5. Nat Commun. 2024. PMID: 39438460 Free PMC article. - Basic Guide for Approaching Drug Delivery with Extracellular Vesicles.
Brezgin S, Danilik O, Yudaeva A, Kachanov A, Kostyusheva A, Karandashov I, Ponomareva N, Zamyatnin AA Jr, Parodi A, Chulanov V, Kostyushev D. Brezgin S, et al. Int J Mol Sci. 2024 Sep 27;25(19):10401. doi: 10.3390/ijms251910401. Int J Mol Sci. 2024. PMID: 39408730 Free PMC article. Review. - Dopey-dependent regulation of extracellular vesicles maintains neuronal morphology.
Park S, Noblett N, Pitts L, Colavita A, Wehman AM, Jin Y, Chisholm AD. Park S, et al. Curr Biol. 2024 Nov 4;34(21):4920-4933.e11. doi: 10.1016/j.cub.2024.09.018. Epub 2024 Oct 7. Curr Biol. 2024. PMID: 39378880 - The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke.
Pawluk H, Tafelska-Kaczmarek A, Sopońska M, Porzych M, Modrzejewska M, Pawluk M, Kurhaluk N, Tkaczenko H, Kołodziejska R. Pawluk H, et al. Biomolecules. 2024 Sep 6;14(9):1130. doi: 10.3390/biom14091130. Biomolecules. 2024. PMID: 39334896 Free PMC article. Review. - Mechanical force of uterine occupation enables large vesicle extrusion from proteostressed maternal neurons.
Wang G, Guasp RJ, Salam S, Chuang E, Morera A, Smart AJ, Jimenez D, Shekhar S, Friedman E, Melentijevic I, Nguyen KC, Hall DH, Grant BD, Driscoll M. Wang G, et al. Elife. 2024 Sep 10;13:RP95443. doi: 10.7554/eLife.95443. Elife. 2024. PMID: 39255003 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R24 OD010943/OD/NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- R01 NS077929/NS/NINDS NIH HHS/United States
- P30 HD071593/HD/NICHD NIH HHS/United States
- T32 GM008339/GM/NIGMS NIH HHS/United States
- R01 AG046358/AG/NIA NIH HHS/United States
- R01 AG047101/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources