The Hippo component YAP localizes in the nucleus of human papilloma virus positive oropharyngeal squamous cell carcinoma - PubMed (original) (raw)
doi: 10.1186/s40463-017-0187-1.
Leanne Clattenburg 2, Shanmugam Muruganandan 2, Martin Bullock 3, Kaitlyn MacIsaac 2, Michael Wigerius 2, Blair A Williams 1, M Elise R Graham 1, Matthew H Rigby 1, Jonathan R B Trites 1, S Mark Taylor 1, Christopher J Sinal 2, James P Fawcett 4 5, Robert D Hart 6
Affiliations
- PMID: 28222762
- PMCID: PMC5320711
- DOI: 10.1186/s40463-017-0187-1
The Hippo component YAP localizes in the nucleus of human papilloma virus positive oropharyngeal squamous cell carcinoma
Faisal Alzahrani et al. J Otolaryngol Head Neck Surg. 2017.
Abstract
Background: HPV infection causes cervical cancer, mediated in part by the degradation of Scribble via the HPV E6 oncoprotein. Recently, Scribble has been shown to be an important regulator of the Hippo signaling cascade. Deregulation of the Hippo pathway induces an abnormal cellular transformation, epithelial to mesenchymal transition, which promotes oncogenic progression. Given the recent rise in oropharyngeal HPV squamous cell carcinoma we sought to determine if Hippo signaling components are implicated in oropharyngeal squamous cell carcinoma.
Methods: Molecular and cellular techniques including immunoprecipiations, Western blotting and immunocytochemistry were used to identify the key Hippo pathway effector Yes-Associated Protein (YAP) 1. Oropharyngeal tissue was collected from CO2 laser resections, and probed with YAP1 antibody in tumor and pre-malignant regions of HPV positive OPSCC tissue.
Results: This study reveals that the Scribble binding protein Nitric Oxide Synthase 1 Adaptor Protein (NOS1AP) forms a complex with YAP. Further, the NOS1APa and NOS1APc isoforms show differential association with activated and non-activated YAP, and impact cellular proliferation. Consistent with deregulated Hippo signaling in OPSCC HPV tumors, we see a delocalization of Scribble and increased nuclear accumulation of YAP1 in an HPV-positive OPSCC.
Conclusion: Our preliminary data indicates that NOS1AP isoforms differentially associate with YAP1, which, together with our previous findings, predicts that loss of YAP1 enhances cellular transformation. Moreover, YAP1 is highly accumulated in the nucleus of HPV-positive OPSCC, implying that Hippo signaling and possibly NOS1AP expression are de-regulated in OPSCC. Further studies will help determine if NOS1AP isoforms, Scribble and Hippo components will be useful biomarkers in OPSCC tumor biology.
Keywords: HPV; Hippo; NOS1AP; Oropharyngeal squamous cell carcinoma; Scribble; YAP; p16.
Figures
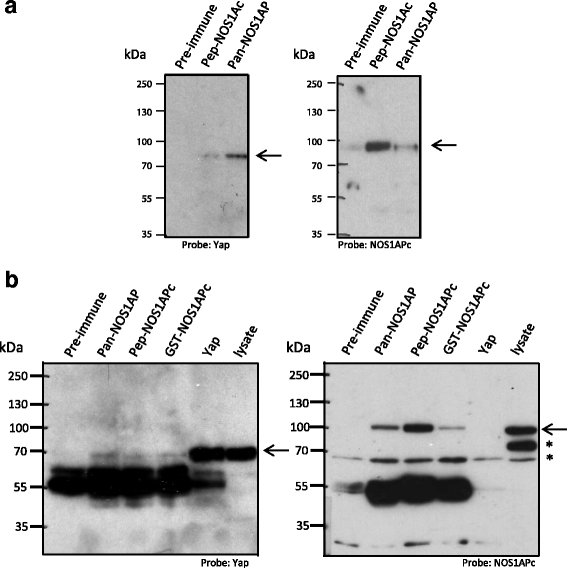
Fig. 1
NOS1AP isoforms associate with YAP. a HEK293T cell lysate was Immunoprecipitated with the antibodies indicated. The resulting blot was probed with anti-YAP (arrow, left panel) and re-probed with a NOS1APc specific antibody (pep-NOS1APc) (arrow, right panel). Note, more YAP associates with the pan-NOS1AP antibody than with the NOS1APc antibody. b Rat brain cell lysate was immunoprecipitated with the indicated antibodies. The resulting blot was probed with anti-YAP antibody (arrow, left panel) and then re-probed with a NOS1APc specific antibody (pep-NOS1APc) (arrow, right panel). Asterisk denote cross reacting bands
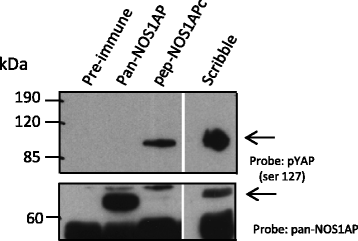
Fig. 2
NOS1APc associates with pYAP. Rat brain cell lysate was immunoprecipitated with the antibodies indicated the resulting blot was probed with the antibodies indicated (upper panel pYAP (ser 127). Lower panel, blot was re-probed with a pan-NOS1AP antibody to identify NOS1APa. Asterisks denote cross reacting band
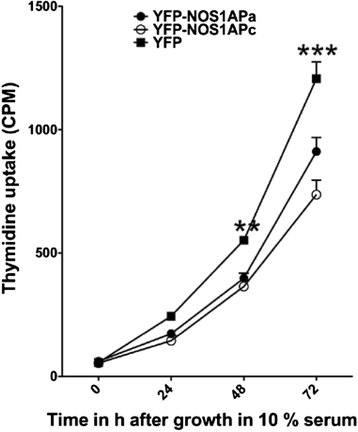
Fig. 3
NOS1AP isoforms affects cellular proliferation. Starved MCF7 cells stably expressing YFP-NOS1APa and YFP-NOS1APc incorporate less radiolabeled thymidine over a 48 and 72h period with 10% serum relative to MCF7 cells stably expressing YFP. Differences were considered significant in Students _t_-test at **P < 0.01, ***p < 0.001
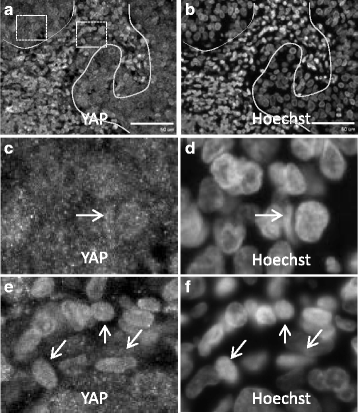
Fig. 4
YAP is activated in HPV+ve-OPSCC. a, b HPV+ve -OPSCC stained with anti-YAP (a) and Hoechst (b). Note: Solid line is tumor margin (a, b). Boxed regions in (a) are expanded in (c) left box and (e) right box. (c, d) Enlarged region ( left box in a) showing Yap (c) and nuclei (d). Note, Yap is mainly localized to small puncta in the cytosol in tumor margins, with some cells showing YAP accumulation in the nucleus (c, d arrow). e, f Enlarged region (right box in a) showing Yap (e) and nuclei (f). Note, Yap is localized in the nucleus in tumor (e, f, arrows). Scale bar = 50um
Similar articles
- NOS1AP Functionally Associates with YAP To Regulate Hippo Signaling.
Clattenburg L, Wigerius M, Qi J, Rainey JK, Rourke JL, Muruganandan S, Sinal CJ, Fawcett JP. Clattenburg L, et al. Mol Cell Biol. 2015 Jul;35(13):2265-77. doi: 10.1128/MCB.00062-15. Epub 2015 Apr 27. Mol Cell Biol. 2015. PMID: 25918243 Free PMC article. - Ultrasensitive detection of oncogenic human papillomavirus in oropharyngeal tissue swabs.
Isaac A, Kostiuk M, Zhang H, Lindsay C, Makki F, O'Connell DA, Harris JR, Cote DW, Seikaly H, Biron VL. Isaac A, et al. J Otolaryngol Head Neck Surg. 2017 Jan 14;46(1):5. doi: 10.1186/s40463-016-0177-8. J Otolaryngol Head Neck Surg. 2017. PMID: 28088212 Free PMC article. - Expression of toll-like receptors in HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma--an in vivo and in vitro study.
Jouhi L, Datta N, Renkonen S, Atula T, Mäkitie A, Haglund C, Ahmed A, Syrjänen S, Grénman R, Auvinen E, Lehtonen S, Hagström J. Jouhi L, et al. Tumour Biol. 2015 Sep;36(10):7755-64. doi: 10.1007/s13277-015-3494-z. Epub 2015 May 5. Tumour Biol. 2015. PMID: 25941114 - The role of Hippo-YAP signaling in squamous cell carcinomas.
Maehama T, Nishio M, Otani J, Mak TW, Suzuki A. Maehama T, et al. Cancer Sci. 2021 Jan;112(1):51-60. doi: 10.1111/cas.14725. Epub 2020 Dec 9. Cancer Sci. 2021. PMID: 33159406 Free PMC article. Review. - Targeting YAP and Hippo signaling pathway in liver cancer.
Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Liu AM, et al. Expert Opin Ther Targets. 2010 Aug;14(8):855-68. doi: 10.1517/14728222.2010.499361. Expert Opin Ther Targets. 2010. PMID: 20545481 Review.
Cited by
- Evaluation of Cervical High-Grade Squamous Intraepithelial Lesions-Correlated Markers as Triage Strategy for Colposcopy After Co-Testing.
Huo X, Sun H, Cao D, Yang J, Peng P, Kong L, Chen F, Shen K, Li S. Huo X, et al. Onco Targets Ther. 2021 Mar 19;14:2075-2084. doi: 10.2147/OTT.S300269. eCollection 2021. Onco Targets Ther. 2021. PMID: 33776454 Free PMC article. - YAP1 activation by human papillomavirus E7 promotes basal cell identity in squamous epithelia.
Hatterschide J, Castagnino P, Kim HW, Sperry SM, Montone KT, Basu D, White EA. Hatterschide J, et al. Elife. 2022 Feb 16;11:e75466. doi: 10.7554/eLife.75466. Elife. 2022. PMID: 35170430 Free PMC article. - The Hippo Pathway and Viral Infections.
Wang Z, Lu W, Zhang Y, Zou F, Jin Z, Zhao T. Wang Z, et al. Front Microbiol. 2020 Jan 23;10:3033. doi: 10.3389/fmicb.2019.03033. eCollection 2019. Front Microbiol. 2020. PMID: 32038526 Free PMC article. Review. - A System Based-Approach to Examine Host Response during Infection with Influenza A Virus Subtype H7N9 in Human and Avian Cells.
Taye B, Chen H, Yeo DS, Seah SG, Wong MS, Sugrue RJ, Tan BH. Taye B, et al. Cells. 2020 Feb 15;9(2):448. doi: 10.3390/cells9020448. Cells. 2020. PMID: 32075271 Free PMC article. - The regulatory networks of the Hippo signaling pathway in cancer development.
Wang M, Dai M, Wang D, Xiong W, Zeng Z, Guo C. Wang M, et al. J Cancer. 2021 Aug 28;12(20):6216-6230. doi: 10.7150/jca.62402. eCollection 2021. J Cancer. 2021. PMID: 34539895 Free PMC article. Review.
References
- Dahlgren L, Dahlstrand HM, Lindquist D, Hogmo A, Bjornestal L, Lindholm J, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112(6):1015–1019. doi: 10.1002/ijc.20490. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources