Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function - PubMed (original) (raw)
. 2017 Mar 1;12(3):e0173004.
doi: 10.1371/journal.pone.0173004. eCollection 2017.
Noora Ottman 1 2 3, Marjolein Meijerink 5 6, Taija E Pietilä 7, Veera Kainulainen 8, Judith Klievink 9 10, Laura Huuskonen 7, Steven Aalvink 1, Mikael Skurnik 9 11, Sjef Boeren 12, Reetta Satokari 9, Annick Mercenier 5, Airi Palva 7, Hauke Smidt 1, Willem M de Vos 1 7 9, Clara Belzer 1
Affiliations
- PMID: 28249045
- PMCID: PMC5332112
- DOI: 10.1371/journal.pone.0173004
Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function
Noora Ottman et al. PLoS One. 2017.
Abstract
Gut barrier function is key in maintaining a balanced response between the host and its microbiome. The microbiota can modulate changes in gut barrier as well as metabolic and inflammatory responses. This highly complex system involves numerous microbiota-derived factors. The gut symbiont Akkermansia muciniphila is positively correlated with a lean phenotype, reduced body weight gain, amelioration of metabolic responses and restoration of gut barrier function by modulation of mucus layer thickness. However, the molecular mechanisms behind its metabolic and immunological regulatory properties are unexplored. Herein, we identify a highly abundant outer membrane pili-like protein of A. muciniphila MucT that is directly involved in immune regulation and enhancement of trans-epithelial resistance. The purified Amuc_1100 protein and enrichments containing all its associated proteins induced production of specific cytokines through activation of Toll-like receptor (TLR) 2 and TLR4. This mainly leads to high levels of IL-10 similar to those induced by the other beneficial immune suppressive microorganisms such as Faecalibacterium prausnitzii A2-165 and Lactobacillus plantarum WCFS1. Together these results indicate that outer membrane protein composition and particularly the newly identified highly abundant pili-like protein Amuc_1100 of A. muciniphila are involved in host immunological homeostasis at the gut mucosa, and improvement of gut barrier function.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
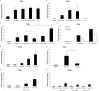
Fig 1. A. muciniphila activates signaling pathways through TLR2 and TLR4.
(A) TLR4 signaling by live A. muciniphila (~107 bacteria/well), A. muciniphila supernatant (~5 μg of protein/well) and LPS isolated from A. muciniphila (concentration corresponds to amount of LPS in ~107 A. muciniphila cells, ~105–106 EU/ml). DMEM: medium control, LPS-EB: positive control (concentration corresponds to amount of LPS in ~107 E. coli cells). (B) TLR2 signaling by live A. muciniphila (~107 bacteria/well), A. muciniphila supernatant (~5 μg of protein/well) and LPS. DMEM; medium control, PAM3CSK4; positive control. (C) TLR2 signaling by live A. muciniphila, L. plantarum, F. prausnitzii and B. breve (~106 bacteria/well). (D) TLR2 signaling in a Transwell system compared to control (i.e. samples not separated from the cell line by a membrane, ~4 × 107 bacteria/well). (E) TLR2 signaling by filtrated supernatant signaling molecules. (F) TLR2 signaling by A. muciniphila bacterial fractions (1 μg of protein/well). (G) TLR2 signaling by A. muciniphila purified protein Amuc_1100 (0.01, 0.1 and 1 μg of protein/well). (H) TLR2 signaling by A. muciniphila purified proteins Amuc_0451, Amuc_0625 and Amuc_2136 (0.1 and 1 μg of protein/well). DMEM; medium control, *, P<0.05 compared to DMEM. All experiments were performed in triplicate and statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD if homogeneity of variance was met or Games-Howell if variance was unequal.
Fig 2. Effect of A. muciniphila, F. prausnitzii and L. plantarum on cytokine production of human PBMCs.
IL-10 (A), TNF-α (B), IL-8 (C) and IL-6 (D) responses of human PBMCs (n = 3 donors) stimulated with A. muciniphila, F. prausnitzii and L. plantarum live cells. IL-10 (E), TNF-α (F), IL-8 (G) and IL-6 (H) responses of human PBMCs (n = 3 donors) stimulated with A. muciniphila, F. prausnitzii and L. plantarum supernatant. *, P<0.05. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD if homogeneity of variance was met or Games-Howell if variance was unequal.
Fig 3. Proteins encoded by the gene cluster Amuc_1098 to Amuc_1102 are found abundantly in a fraction enriched for membrane and cell-envelope proteins.
(A) Number of A. muciniphila membrane and cell-envelope proteins detected with LC-MS/MS in two different fractions from a sucrose density-gradient separation method. (B) Amuc_1098 to Amuc_1102 gene cluster. (C) Abundance of proteins found in Fraction 1 vs. Fraction 2. Relative abundances of the proteins are presented on a log10 scale. A log10 relative abundance of 3.5 represents proteins that were not detected or were under the detection limit.
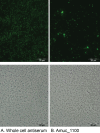
Fig 4. Amuc_1100 is located on the outer membrane of A. muciniphila.
Immunofluorescence staining of A. muciniphila cells with whole cell antiserum (A) or anti-Amuc_1100 (B) and Alexa-488-conjugated secondary IgG. Phase-contrast images of the same microscopic fields are shown below. Pictures were cropped from the original image.
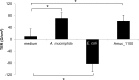
Fig 5. A. muciniphila and outer membrane protein Amuc_1100 increase the development of transepithelial electrical resistance.
The impact of A. muciniphila, purified protein Amuc_1100 (0.05 μg/ml) or E. coli on the TEER development of Caco-2 monolayer after 24 h of stimulation. Mean and standard deviations from three parallel wells are shown. Significant differences (p < 0.05) in the TEER values as compared to control (growth medium without bacteria) at 24 h are indicated with an asterix. Statistical analysis was performed by one-way analysis of variance (ANOVA).
Similar articles
- Action and function of Akkermansia muciniphila in microbiome ecology, health and disease.
Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Ottman N, et al. Best Pract Res Clin Gastroenterol. 2017 Dec;31(6):637-642. doi: 10.1016/j.bpg.2017.10.001. Epub 2017 Oct 13. Best Pract Res Clin Gastroenterol. 2017. PMID: 29566906 Review. - The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling.
Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. Wang J, et al. Food Funct. 2021 Apr 26;12(8):3597-3610. doi: 10.1039/d1fo00115a. Food Funct. 2021. PMID: 33900345 - Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids.
Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. Lukovac S, et al. mBio. 2014 Aug 12;5(4):e01438-14. doi: 10.1128/mBio.01438-14. mBio. 2014. PMID: 25118238 Free PMC article. - Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145.
Wade H, Pan K, Duan Q, Kaluzny S, Pandey E, Fatumoju L, Saraswathi V, Wu R, Harris EN, Su Q. Wade H, et al. J Biomed Sci. 2023 Jun 7;30(1):38. doi: 10.1186/s12929-023-00935-1. J Biomed Sci. 2023. PMID: 37287024 Free PMC article. - Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders.
Macchione IG, Lopetuso LR, Ianiro G, Napoli M, Gibiino G, Rizzatti G, Petito V, Gasbarrini A, Scaldaferri F. Macchione IG, et al. Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):8075-8083. doi: 10.26355/eurrev_201909_19024. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599433 Review.
Cited by
- Exploring alterations of gut/blood microbes in addressing iron overload-induced gut dysbiosis and cognitive impairment in thalassemia patients.
Suparan K, Trirattanapa K, Piriyakhuntorn P, Sriwichaiin S, Thonusin C, Nawara W, Kerdpoo S, Chattipakorn N, Tantiworawit A, Chattipakorn SC. Suparan K, et al. Sci Rep. 2024 Oct 23;14(1):24951. doi: 10.1038/s41598-024-76684-4. Sci Rep. 2024. PMID: 39438708 Free PMC article. - Faecal microbiota transplantation as an elixir of youth.
Haifer C, Paramsothy S, Leong RW. Haifer C, et al. Hepatobiliary Surg Nutr. 2020 Aug;9(4):488-489. doi: 10.21037/hbsn.2019.11.17. Hepatobiliary Surg Nutr. 2020. PMID: 32832499 Free PMC article. No abstract available. - Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system (Review).
Jiang H, Zhang Q. Jiang H, et al. Oncol Lett. 2024 Jan 5;27(2):87. doi: 10.3892/ol.2024.14221. eCollection 2024 Feb. Oncol Lett. 2024. PMID: 38249807 Free PMC article. Review. - Oral and Gut Microbial Dysbiosis and Non-alcoholic Fatty Liver Disease: The Central Role of Porphyromonas gingivalis.
Wang T, Ishikawa T, Sasaki M, Chiba T. Wang T, et al. Front Med (Lausanne). 2022 Mar 2;9:822190. doi: 10.3389/fmed.2022.822190. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35308549 Free PMC article. Review. - Effects of Amazake Produced with Different Aspergillus on Gut Barrier and Microbiota.
Nakano H, Setoguchi S, Kawano K, Miyagawa H, Sakao K, Hou DX. Nakano H, et al. Foods. 2023 Jun 30;12(13):2568. doi: 10.3390/foods12132568. Foods. 2023. PMID: 37444313 Free PMC article.
References
- Rescigno M. Mucosal immunology and bacterial handling in the intestine. Best practice & research Clinical gastroenterology. 2013;27(1):17–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources